2024 AIChE Annual Meeting
(569bv) Optimizing the Greener Electrochemical Synthesis of Dimethyl Carbonate from CO2
Authors
To address this utilization challenge, researchers have begun to explore sustainable chemical production from CO2; for example, Lee et al.2 recently reported the electrochemical synthesis of dimethyl carbonate (DMC) using CO2. DMC is the simplest organic carbonate with uses across the chemical industry due to its low toxicity, polarity, and viscosity.3 Typical electrochemical reactions, however, are limited by the use of rare and expensive catalyst and electrode materials that degrade during the reaction.
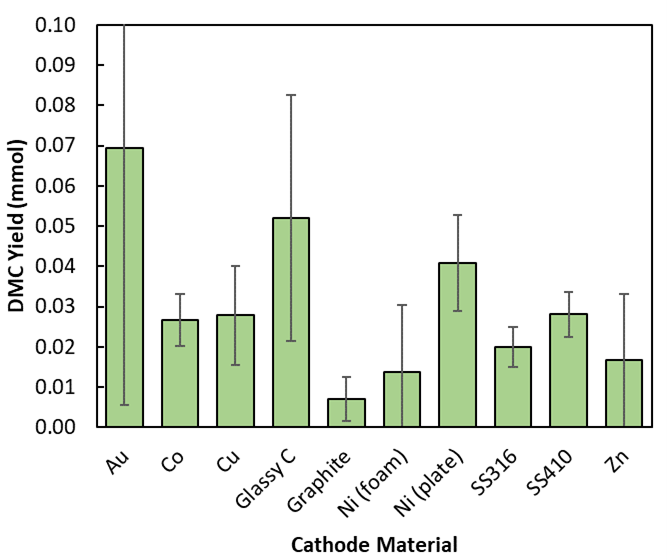
This work aims to explore the effectiveness and longevity of abundant electrode materials on the electrochemical production of DMC. First, 10 materials were tested as cathodes (Figure 1) to compare resultant DMC yields. Of these, glassy carbon (GC) has emerged as the most effective cathode, behind gold. As one of the most common carbon-based electrodes, GC is known for its electrochemical stability and high overpotential for oxygen and hydrogen evolution.4 Furthermore, the chemical stability and impermeability of GC has been explored through analysis of catalyst deposition and productivity over time, preliminarily showing an increase in electrode lifetime compared to gold due to a reduction in catalyst adsorption that blocks active sites and hinders the reaction.
- Lee, H. et al. IPCC, Geneva, Switzerland. 2023.
- Lee, K.M. et al. Nature Energy 2021, 6(7), 733-741.
- Santos, B. A. V. et al. ChemBioEng Reviews 2014, 1 (5), 214-229.
- Bystron, T. et al. 2019, 299, 963-970.