The urgent need for carbon-free energy solutions to mitigate greenhouse gas emissions and promote sustainable development has driven extensive research into hydrogen production. Hydrogen, once primarily an intermediate chemical, is now recognized as a clean fuel. However, conventional methods like natural gas reforming result in high CO
2 emissions. Alternative technologies, such as water electrolysis for green hydrogen, face limitations. As global energy demand rises, the dominance of fossil fuels contributes significantly to CO
2 emissions and prompts a shift towards renewable energy sources. Various methodologies for hydrogen sulfide (H
2S) utilization have been explored, with emerging approaches like the hydrogen sulfide-iodine (H
2S-I) thermochemical cycle showing promise for cleaner hydrogen production but requiring further development for commercial use.
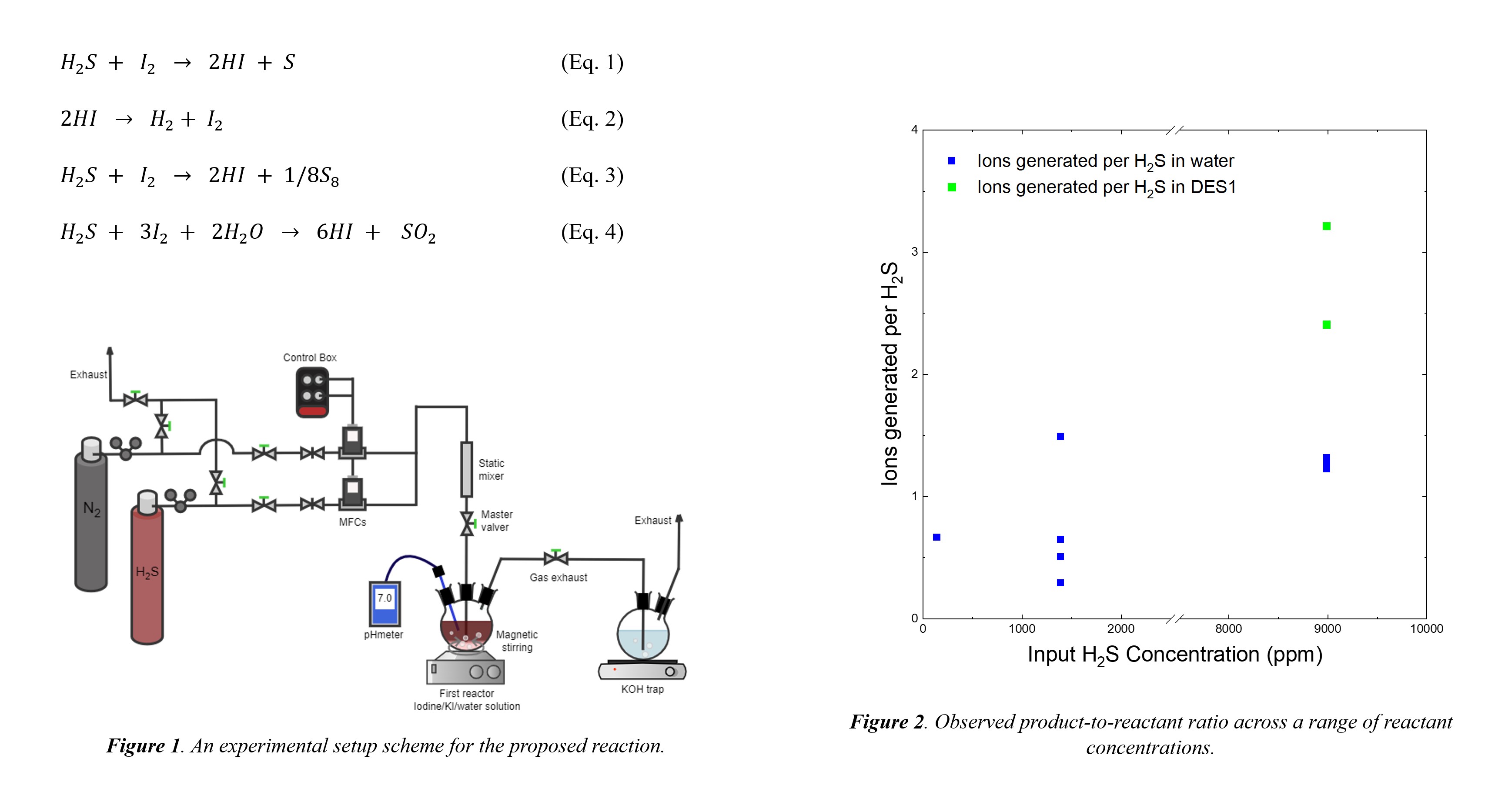
The H2S-I cycle involves reactions between H2S and iodine to produce hydrogen and sulfur (Equations 1 and 2), with potential challenges in isolation and scalability. Water-based approaches offer thermodynamic advantages but face cost and energy challenges. Deep eutectic solvents (DESs) show promise for H2S absorption, with DESs offering advantages in cost and scalability. Experimental studies (Figure 1) are underway to compare conventional water-based processes with DES-based methods to enhance conversion efficiency. Initial results suggest concentration-dependent hydrogen iodide yields (HI) (equations 3 and 4) based on an H2S high-concentration or low-concentration regime in aqueous media, with DES showing potential for increased conversion (Figure 2).
Different conditions, such as H2S concentration, solvent used, and temperature, are being explored to determine which are more suitable for an effective conversion and production of hydrogen. Current findings have shown that temperature increases can influence HI production, considering a range between room temperature and 80°C. Implementing the H2S-I cycle presents an environmentally friendly approach to cleaner energy production and toxic waste disposal. This research contributes valuable insights into sustainable hydrogen production methods and aims to integrate them into existing industrial processes.