As pharmaceutical manufacturers are looking for ways to shift toward continuous manufacturing, one of the biggest challenges has been the continuous separation and purification of active pharmaceutical ingredients (APIs) or intermediates after crystallization or reactive crystallization. A significant gap exists between continuous crystallization and the availability of corresponding filtration equipment
1. Keeping this obstacle in mind, a continuous rotary filter was developed, and its design space was investigated using a commercially available API
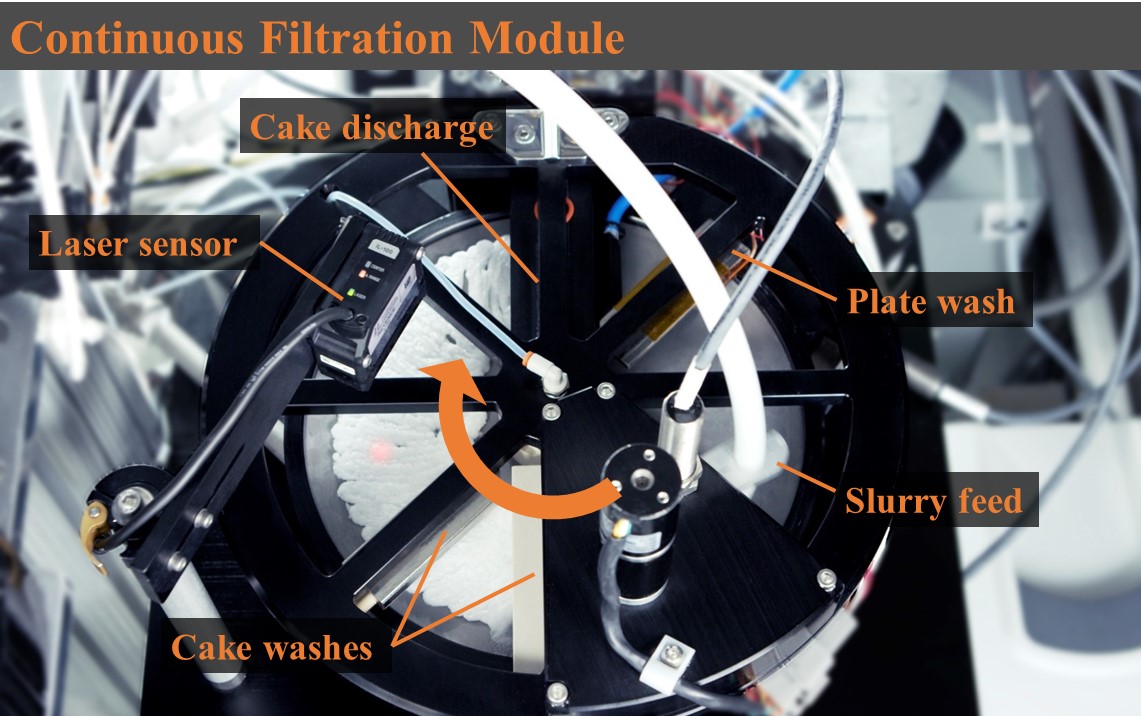
2. The filtration unit consists of a rotatable plate that receives a continuous flow of feed material. The feed was then evenly distributed along the radius of the plate, forming a thin cake. The cake is continuously filtered, washed, and removed from the plate. An additional plate wash continually cleans the filter medium, enabling long-term operations. On the stand-alone unit, a slurry containing the selected API and two main impurities was processed. Process parameters including cake residence times and cake wash rates were investigated. By operating the filter continuously, the process can be adjusted to minimize the cake resistance, therefore facilitating efficient filtration performance. Process parameters for traditional filtration study such as cake resistance and filtration time became constant throughout the continuous operation. The continuous filter was then incorporated into an integrated continuous manufacturing (ICM) line at pilot scale, where it processed the slurry from a crystallizer and then transferred the wet cake into the downstream process
3. In both settings, the rotary filter isolated and purified the cake to the required quality specifications, proving its effectiveness and robustness during continuous operations. The process design has been further improved to allow technology transfer for GMP manufacturing. An advanced filter unit operation is designed with GMP compliant approaches, and the cleaning-in-place (CIP) capacity is incorporated. We report the commissioning of a pilot-scale filter with high containment capabilities, which can be used in laboratory or piloting environments to develop manufacturing technologies prior to commercial implementation. This advancement expands R&D activities for continuous manufacturing to include potent compounds isolations and purification, enabling a broader scope for pharmaceutical development.
References
(1) Cote, A.; Erdemir, D.; Girard, K. P.; Green, D. A.; Lovette, M. A.; Sirota, E.; Nere, N. K. Perspectives on the Current State, Challenges, and Opportunities in Pharmaceutical Crystallization Process Development. Cryst Growth Des 2020, 20 (12), 7568–7581. https://doi.org/10.1021/acs.cgd.0c00847.
(2) Wu, W.; Sayin, R.; Shvedova, K.; Born, S. C.; Testa, C. J.; Yeole, S. S.; Censullo, A. S.; Srivastava, A. K.; Ramnath, A.; Hu, C.; Takizawa, B.; O’Connor, T. F.; Yang, X. B.; Ramanujam, S.; Mascia, S. A Continuous Rotary Filtration for the Separation and Purification of an Active Pharmaceutical Ingredient. Org Process Res Dev 2023. https://doi.org/10.1021/acs.oprd.3c00263.
(3) Testa, C. J.; Hu, C.; Shvedova, K.; Wu, W.; Sayin, R.; Casati, F.; Halkude, B. S.; Hermant, P.; Shen, D. E.; Ramnath, A.; Su, Q.; Born, S. C.; Takizawa, B.; Chattopadhyay, S.; O’Connor, T. F.; Yang, X.; Ramanujam, S.; Mascia, S. Design and Commercialization of an End-to-End Continuous Pharmaceutical Production Process: A Pilot Plant Case Study. Org Process Res Dev 2020, 24 (12), 2874–2889. https://doi.org/10.1021/acs.oprd.0c00383.