2024 AIChE Annual Meeting
(537c) Structure and CO2 Capture Properties of Polyanionic Films Containing Perfluoroalkyl Sulfonylimide
Authors
Vinh Bui - Presenter, University at Buffalo
Owen Lee, University of Colorado
Leiqing Hu, University At Buffalo
Ryan Hayward, University of Colorado Boulder
Haiqing Lin, University of Buffalo, State University of New Yor
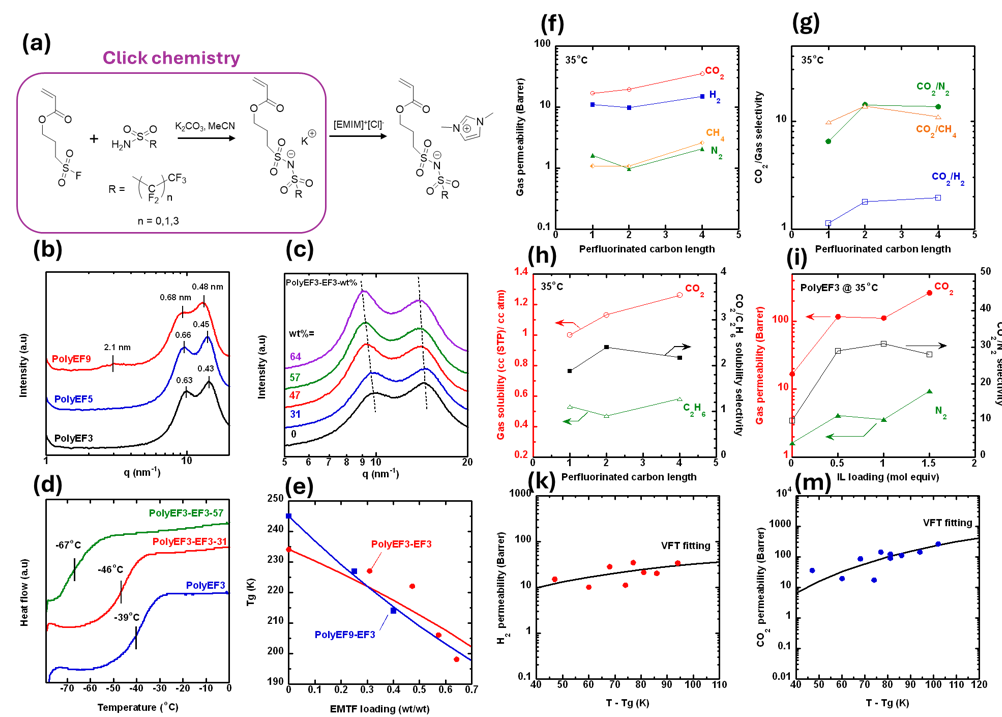
Poly(ionic liquids) (PolyILs) with excellent mechanical stability and strong CO2 affinity have emerged as an exciting platform for membrane carbon capture. This study aims to systematically evaluate the effect of fluorine content on structure characteristics and CO2/gas separation properties of novel anionic PolyIL membranes. First, ionic liquid of 1-ethyl-3-methylimidazolium 3-acryloxypropylsulfonyl(-perfluoroalkyl-[sulfonimide]) with 3, 5 or 9 fluorine groups (or EF3, EF5 and EF9, respectively) were prepared via Sulfur(VI) Fluoride Exchange (SuFEx) click chemistry. These ILs can be cross-linked with 2 mol% of poly(ethylene glycol) diacrylate (PEGDA) to obtain mechanically stable PolyILs (ie. PolyEF) membrane for CO2 separation application. Characterization of these PolyEF membranes is systematically investigated via FTIR, DSC, TGA, XRD, gas sorption and permeation apparatus. For example, increasing the flourine content between PolyEF3 and PolyEF9 increases the glass transition temperature from -39 to -12oC, and increases the counterion d-spacing from 0.43 to 0.48 nm, respectively, and enhances CO2 permeability from 17 to 35 Barrer, and CO2/N2 from 10 to 17, respectively. Interestingly, free EF ionic liquid loaded PolyEFs exhibit reduction in Tg but enhancement in counterion d-spacing and simultaneous improvement in gas permeability and CO2/gas selectivity for all samples. For instance, addition of 57wt% of EF3 in PolyEF3 decreases the Tg from -39 to -67oC, respectively, but enhances CO2 permeability from 17 to 260 Barrer, and CO2/N2 from 10 to 28, respectively. Direct correlation between polymer transition temperature and gas transport properties can be satisfactorily described by the Vogel-Fulcher-Tammann (VFT) equation. This systematic study highlights the attractive CO2/gas separation properties of polyanionic liquid membranes, providing insight into the design of polyILs membranes for practical CO2 capture applications.