2024 AIChE Annual Meeting
(536b) Optimizing the Combustion Synthesis of FeAlxOy Catalysts for Microwave-Assisted Thermocatalytic Dehydrogenation of Fossil Fuels.
Authors
Laura A. Martinez Espinoza, The University of Texas at El Paso
Evgeny Shafirovich, Purdue University
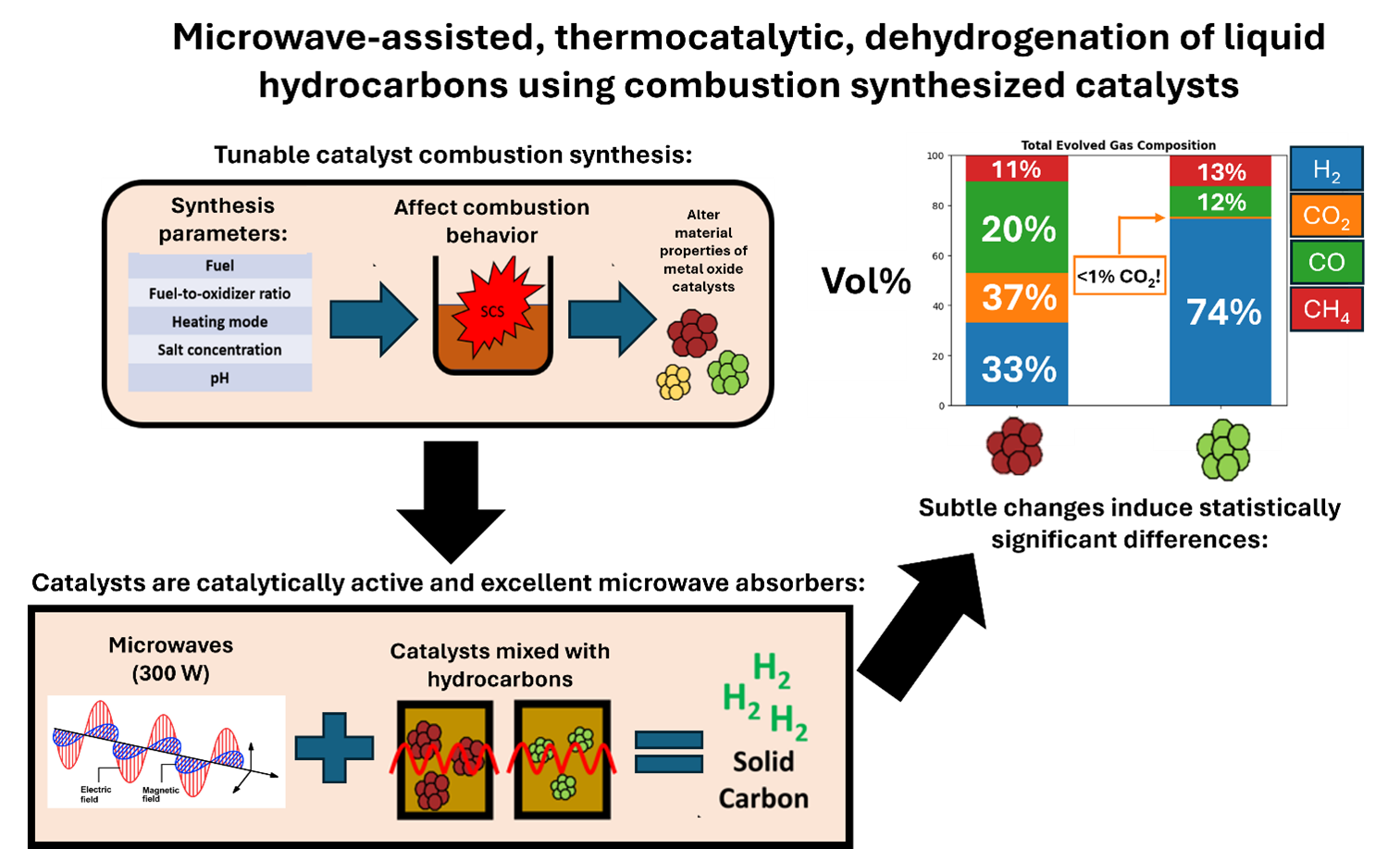
The growing demand for hydrogen (H2) requires the development of clean and energy-efficient technologies for its synthesis. Microwave-assisted thermocatalytic dehydrogenation of fossil fuels has demonstrated the potential to produce H2 with high yield and selectivity, and simultaneously generate valuable nanostructured carbon byproducts. In prior work, iron-based alumina (FeAlxOy) catalysts for this process were made via solution combustion synthesis (SCS). However, the effect of SCS parameters on the dehydrogenation performance is not well understood. The present study investigates this by varying the SCS fuel, Fe:Al molar ratio, and heating mode. The results show subtle changes of these parameters can result in significant differences in the phase composition, specific surface area, and microwave absorbing properties of FeAlxOy, which all affect microwave-assisted dehydrogenation. Notably, H2 selectivity can be increased from 30% to 74%. Statistical testing determined that the SCS fuel used was the most significant SCS parameter affecting dehydrogenation performance.