CO
2 conversion to synthetic fuels and chemicals is a rapidly growing research field where many different reaction pathways are being investigated, including electrochemical, photochemical, biological, and thermocatalytic conversions. Thermocatalytic conversion includes, among others, methanation and reverse water gas shift reactions. Reverse water gas shift (RWGS) is a key reaction for thermo-catalytic reduction, as the CO/H
2 mixture produced (syngas) can be utilized as feedstock for various downstream reactions (including Fischer-Tropsch synthesis). Due to the endothermic nature of RWGS, high temperatures are required to achieve high, practically relevant conversion of CO
2 (> 50-60%). However, conducting catalytic reactions at elevated temperatures cause catalyst deactivation through coking and sintering. Thus, there is a strong need to develop catalysts that are highly active, as well as selective to CO, at relatively lower temperatures.
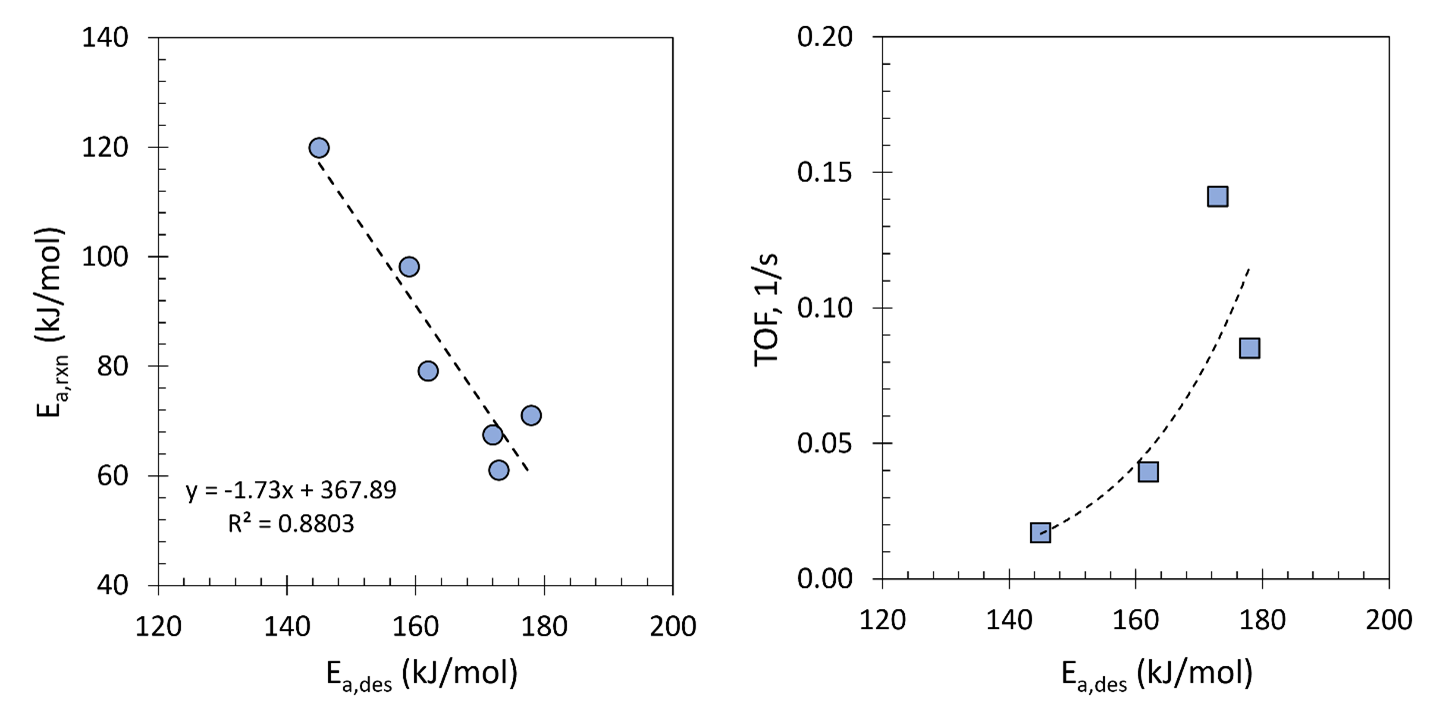
In this study, copper-doped ceria (CuCeO2) catalysts with 0-26.5 Cu/(Cu+Ce) at% were synthesized via the reverse microemulsion method. X-ray diffraction analysis of freshly synthesized and spent catalysts showed no separate phase of copper or copper oxide, indicating that Cu was incorporated into the CeO2 lattice replacing Ce. Temperature programmed desorption showed that the activation energy of CO2 desorption increased for higher Cu loadings, indicating stronger CO2 adsorption. This phenomenon was attributed to enhanced formation of oxygen vacancies due to Cu doping. X-ray photoelectron spectroscopy confirmed the enhanced generation of oxygen vacancies due to Cu incorporation. Catalytic performance evaluation with the H2/CO2 feed in the 300-600 °C range showed that all catalysts were 100% selective to CO generation, with higher Cu loadings resulting in CO2 conversion close to equilibrium values at 500-600 °C. The activation energy of the reaction, determined through reaction tests, exhibited a linear relationship with the activation energy of CO2 desorption. The relationship between these two energy barriers is explored, providing valuable insights into the mechanism of RWGS activity enhancement.