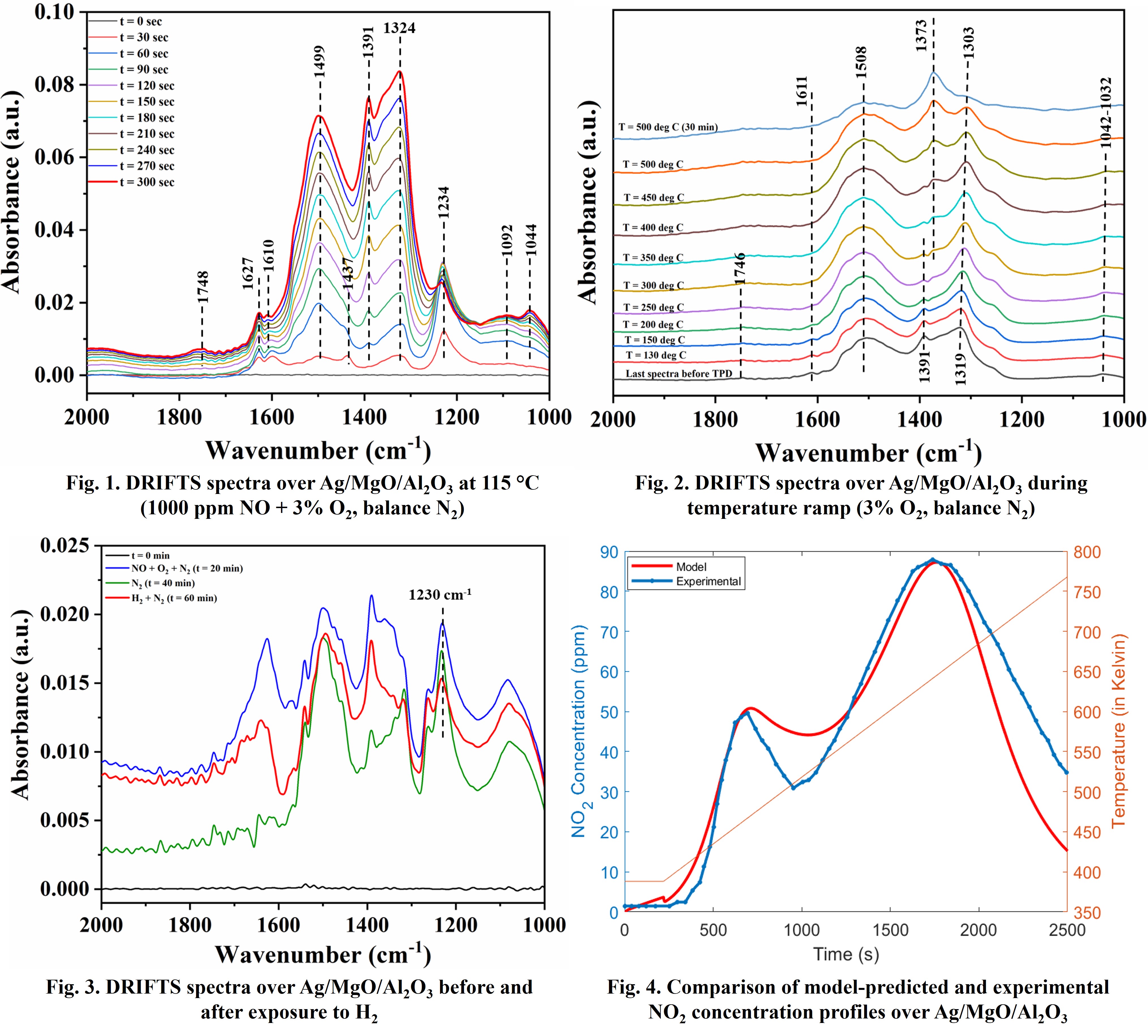
Dual-functional Ag/MgO/Al
2O
3 catalyst is developed, which functions as a passive NO
x adsorber at low temperatures (115 ℃) and a NO
x reduction catalyst at higher temperatures. A combination of bench-scale reactor studies and in-situ DRIFTS experiments are used to propose the mechanism for NO
x adsorption and reduction. It is found that NO gets adsorbed in the form of nitrites (NO
2-) and nitrates (NO
3-) at low temperatures (Fig. 1). The species (1234 cm
-1) are formed at early times, and the peak intensity of species (1499 and 1324 cm
-1) increases at later times. The intensities of some of the nitrate peaks (1499 cm
-1) are significantly higher over Ag/MgO/Al
2O
3 as compared to Ag/Al
2O
3, MgO, Al
2O
3, and the bare MgO-Al
2O
3 support, thus suggesting the significant role of metal-support interactions in enhancing the amount of NO
x storage over Ag/MgO/Al
2O
3. Adsorption experiments are followed by temperature ramp experiments, and the DRIFTS peaks at 1508, 1373, and 1319 cm
-1 are present even at 500 ℃ (Fig. 2). The presence of H
2 significantly increases the amount of NO
x storage and is explained by the conversion of NO
2- to NO
3- species (Fig. 3). Even though the adsorbed species are the same in the presence and absence of O
2, the formation of nitrites is more in the presence of O
2. Moreover, the same adsorbed species are formed with NO and NO
2, which is consistent with reactor experiments where similar adsorption/desorption features are observed in the presence of these two species. Based on the insights obtained, a kinetic model is developed to predict the spatio-temporal profiles of NO and NO
2 over Ag/MgO/Al
2O
3 (Fig. 4). In the full manuscript, the mechanistic insights in the presence of C
3H
6 and NH
3 reductants will be presented, and the effect of replacing Ag with Cu will be discussed.