Nitrous oxide (N
2O) is a potent greenhouse gas and ozone-depleting substance emitted from industrial and automotive processes such as NH
3 combustion, threatening to erase environmental benefits of using NH
3 as a decarbonized fuel. Thus, new catalytic technologies are needed to mitigate N
2O. Fe-zeolites are active for NO
x selective catalytic reduction (SCR) with NH
3[1] and have been shown to activate N
2O for partial methane oxidation,
[2] suggesting Fe-zeolites are promising candidates for N
2O-SCR. However, the kinetics and mechanisms of N
2O-SCR by Fe-zeolites remain incompletely understood.
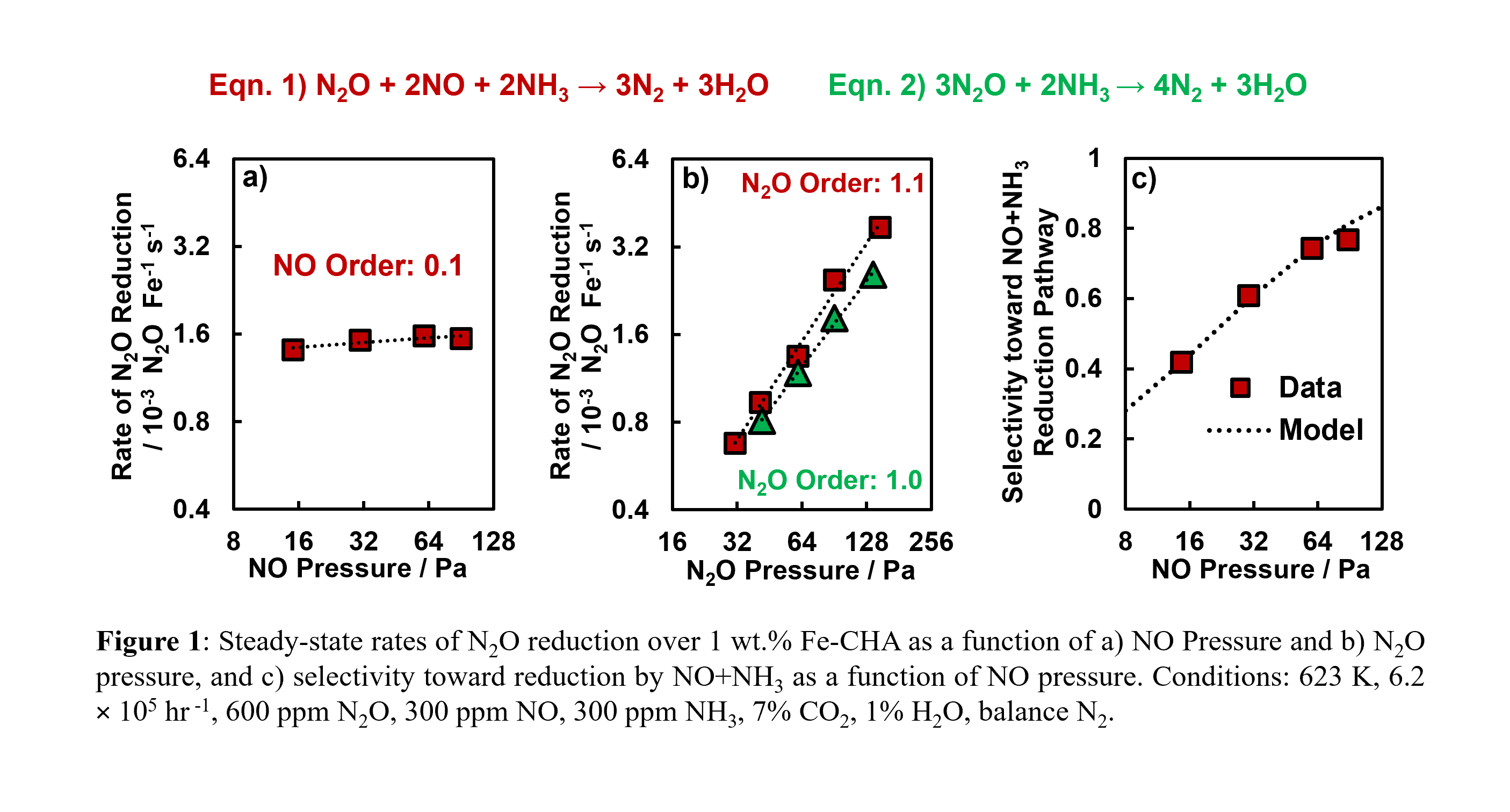
Here, we prepared a 1 wt.% Fe-chabazite (Si/Al = 12) to investigate the redox pathways of N2O-SCR by NH3 and NO. Temperature programmed titration measurements confirm Fe is present entirely as extraframework cations. Steady-state N2O reduction rates were measured in a differential plug flow reactor. At 623 K, N2O reduction occurs via two competitive, parallel pathways, involving either NH3-only (Eqn. 2) or NO+NH3 (Eqn. 1) as reductants. We varied reaction gas compositions (in either N2O/NO/NH3, or N2O/NH3) and temperature to gain insights of N2O-SCR rates and reaction pathway selectivities. N2O-SCR rates in both gas mixtures are 0 order in NO (Fig. 1a), 1st order in N2O (Fig. 1b), and display similar apparent activation energies (~175 kJ mol-1), consistent with both redox cycles having the same rate-limiting oxidation step. However, selectivity towards Eqn. 1 increases with NO pressure (Fig. 1c), because reduction by NO+NH3 has an intrinsically 1st order dependence on NO. Selectivity toward Eqn. 1 decreases with temperature, suggesting the NO+NH3 pathway has a lower apparent activation energy than the NH3-only pathway. This work elucidates the kinetics of disparate reduction pathways of N2O-SCR, aiding design of catalysts for N2O abatement in applications including NH3 engines.
1) Yasumura, et al. ACS Catal. 2022, 9983–9993. 2) Bols, et al. J. Am. Chem. Soc., 2018, 140.