Methane pyrolysis (CH
4(g)→C(s)+2H
2(g)) in a molten media is an emerging technology to produce CO
2-free hydrogen and to overcome catalyst deactivation issues associated with solid catalysts. Molten salts, which are potential media for methane pyrolysis, often exhibit weak catalytic activity, which can be enhanced by dispersing active metals into them. Our investigation focuses on methane dehydrogenation reactions and C-C coupling reactions, utilizing various metal-dispersed (Ni, NiB, Cu, CuB) molten salt (NaBr) systems through Car-Parrinello Molecular Dynamics simulations. Our main objective was to enhance the production of more valuable C
2 products over carbon.
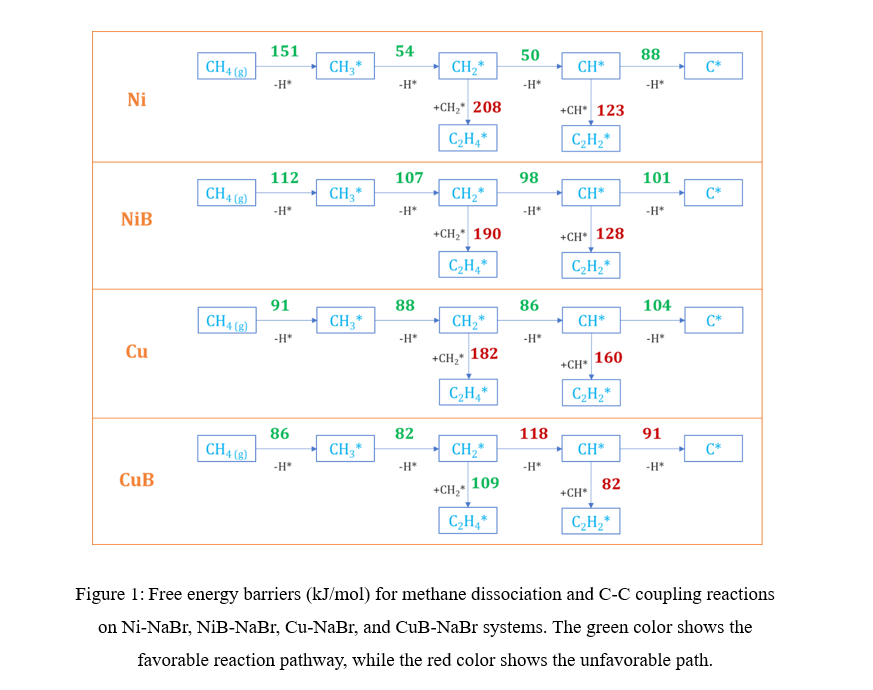
The free energy barrier for CH4 dehydrogenation to CH3 and H on the Ni-NaBr, NiB-NaBr, Cu-NaBr, and CuB-NaBr systems were found to be 151 kJ/mol, 112 kJ/mol, 91 kJ/mol, and 86 kJ/mol respectively. For NiB and CuB dispersed systems, the improved activity is attributed to boron doping, whereas for the Cu system, it is due to Cu cluster formation. Our simulations revealed that carbon, generated as a byproduct, diffuses into the bulk of the dispersed Ni and Cu, leading to their deactivation. Conversely, the boron-doped systems impede this diffusion, making them promising candidates for averting catalyst deactivation. Furthermore, we investigated C-C coupling reactions on these systems. In the case of the Ni, NiB, and Cu dispersed systems, the free energy barrier for C-C coupling reactions (such as CH2-CH2 and CH-CH coupling) was higher than the barrier for CHx (x = 2, 1) dehydrogenation. Hence, these systems do not facilitate the C-C coupling reactions. Interestingly, in the case of the CuB dispersed system, the free energy barrier for the coupling of CH2 fragments (109 kJ/mol) is lower than that for the CH2 dehydrogenation barrier (118 kJ/mol). Consequently, the CuB dispersed system promotes the CH2-CH2 coupling reaction over the CH2 dehydrogenation, making it a good candidate for producing C2 products.