2024 AIChE Annual Meeting
(509f) Computational Investigation into Supported and Inverted Cu/ZrO2 Catalysts for Selective Conversion of CO2 to Methanol
Authors
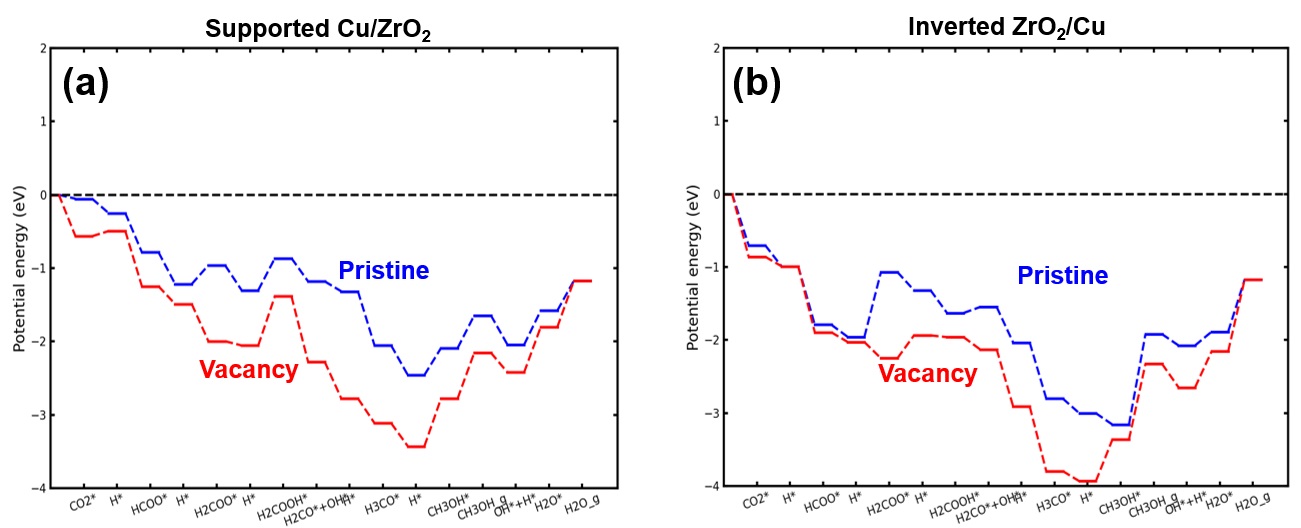
In this work, we utilize Density Functional Theory (DFT) calculations to study atomistic models representing supported and inverted Cu-ZrO2 systems. We elucidate charge transfer characteristics as well as thermodynamic stability of both systems under operando conditions. We find that the formation of oxygen vacancies, prominently around interfacial locations, is most feasible under hydrogen pre-treatment conditions, prior to introducing CO2 at the inlet. We compute adsorption energies of intermediates relevant to CO2 hydrogenation and demonstrate that the presence of oxygen vacancies leads to additional stabilization of all species on both systems, further enabling the mechanistic pathways for hydrogenation (Figure 1). The selectivity performance of inverted systems towards is shown to be higher than supported system, owing to the excess stabilization of adsorbed CO on the former compared to the latter.
- Yuan et al. Chemical Engineering Communications (2022).
- Li et al. ACS Catal 9, 7840–7861 (2019).
- Rui et al. Ind Eng Chem Res 60, 18900–18906 (2021).
- Wu et al. Nat Commun 11, (2020).
- Duyar et al. Angewandte Chemie 130, 15265–15270 (2018).