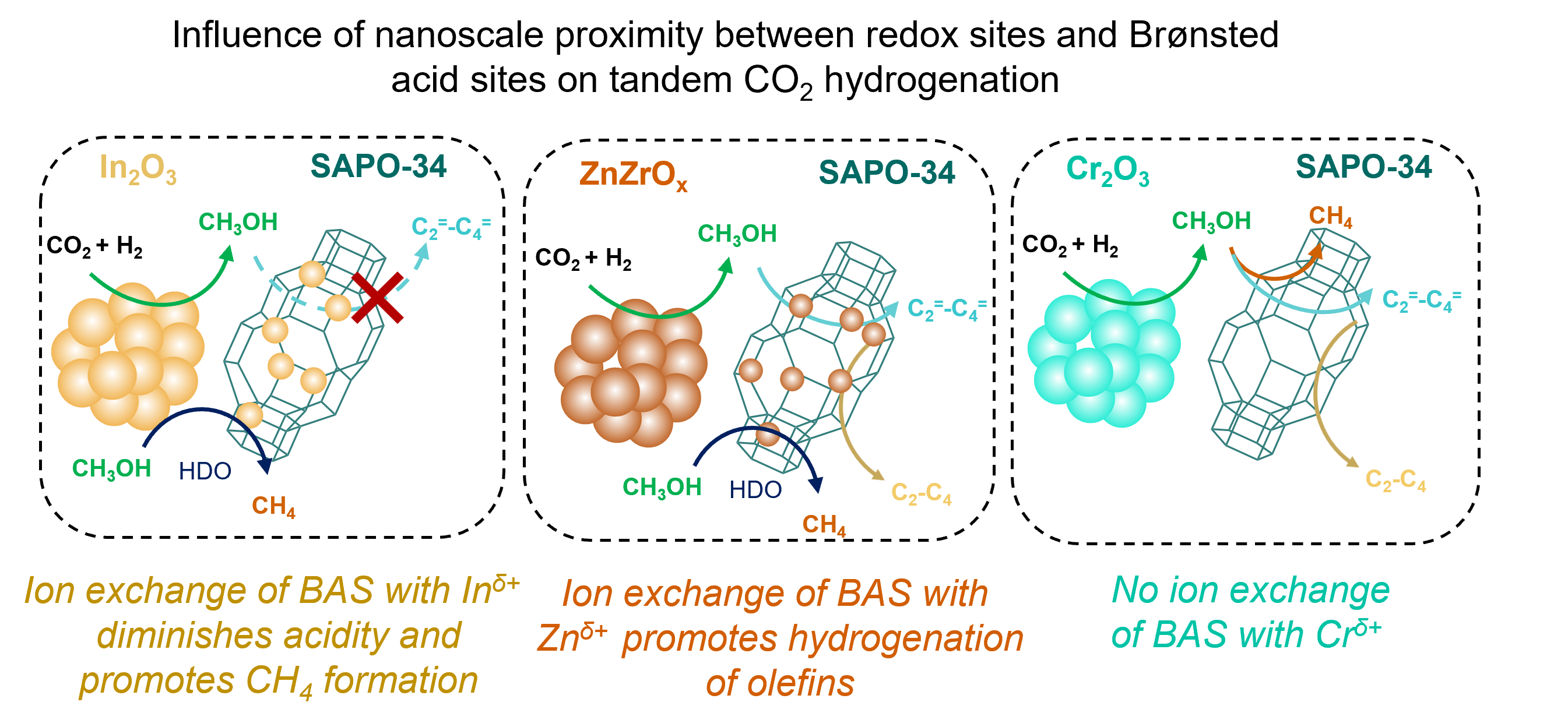The influence of metal oxides on methanol (CH
3OH)-mediated conversion of CO
2 to olefin was investigated using SAPO-34 (a silicoaluminophospate) with three representative metal oxides (e.g., In
2O
3, ZnZrO
x, Cr
2O
3). The placement of redox sites of metal oxides and Brønsted acid sites (BAS) of SAPO-34 was modulated to probe their proximity-dependent reactivity. Analysis of the intermediate CH
3OH transfer rates revealed that CH
3OH transfer could be faster over intrapellet admixtures (distance between redox and BAS ~255-1500 nm) as compared to interpellet admixtures (~200-325 µm), resulting in a higher space-time yield (STY) of hydrocarbons over ZnZrO
x/SAPO-34 (17x10
-5 and 11x10
-5 mol
C g
cat-1 min
-1 over intrapellet and interpellet admixtures, respectively) and Cr
2O
3/SAPO-34 (1.4x10
-5 and 1.2x10
-5 mol
C g
cat-1 min
-1 over intrapellet and interpellet admixtures, respectively) at 400°C and 3 MPa. However, for intrapellet In
2O
3/SAPO-34, the catalytic activity was found to diminish yielding mostly CH
4(95%). While powder X-ray diffraction (PXRD), Ar physisorption, and transmission electron microscopy (TEM) revealed no change to the SAPO-34 structure in intrapellet admixtures, X-ray photoelectron spectroscopy (XPS) revealed the occurrence of solid-state ion exchange (SSIE) between BAS of SAPO-34 with In
δ+ and Zn
δ+ ions from In
2O
3 and ZnZrO
x, respectively. Although ion-exchanged In
δ+ species diminished the acidity of SAPO-34 and inhibited the propagation of olefin and aromatic cycles in the hydrocarbon pool mechanism, it promoted CH
4 formation
via CH
3OH hydrodeoxygenation (HDO). Interestingly, ion-exchanged Zn
δ+ species in intrapellet admixture enhanced secondary hydrogenation of olefins and exhibited ~5 higher paraffin-to-olefin ratio in HCP, as compared to its interpellet admixture. Conversely, ion exchange was not observed in Cr
2O
3/SAPO-34 systems. The likelihood of the formation of cationic species from the metal oxides was further demonstrated in terms of metal vacancy formation energies, which was found to be consistent with our reactivity data (ZnO (1.2 eV) >In
2O
3 (4.6 eV) > Cr
2O
3 (5.8 eV) >ZrO
2 (20.43 eV)).