Environmental degradation persists and is worsened by the accumulation of waste plastics, and biomass like bagasse, corn cob, etc., highlighting the urgent need for innovative solutions. Traditional methods of managing these materials include landfill disposal, incineration, and mechanical recycling. These methods have contributed to the degradation of ecosystems, air and water pollution, and worsening climate change. Plastic waste pollutes landfills and oceans, releasing toxins and disrupting marine life, while unregulated biomass disposal leads to deforestation, soil degradation, and greenhouse gas emissions.
The co-gasification of plastics and biomass provides various advantages, including a high H/C ratio of plastic that counterbalances the higher oxygen concentration in biomass. As a result, there is an increase in hydrogen yield, a reduction in tar generation, and an improvement in syngas quality. The use of biomass, which is renewable and carbon-neutral, complements the energy content of plastics in cogasification, resulting in a higher calorific value of the feedstock mixture and greater energy yields per unit of input. Including biomass in cogasification processes supports sustainability goals by decreasing dependence on fossil fuels and mitigating greenhouse gas emissions. Syngas generated from biomass can be considered carbon-neutral since the carbon dioxide released during combustion or utilization is compensated by the carbon dioxide absorbed during biomass growth.
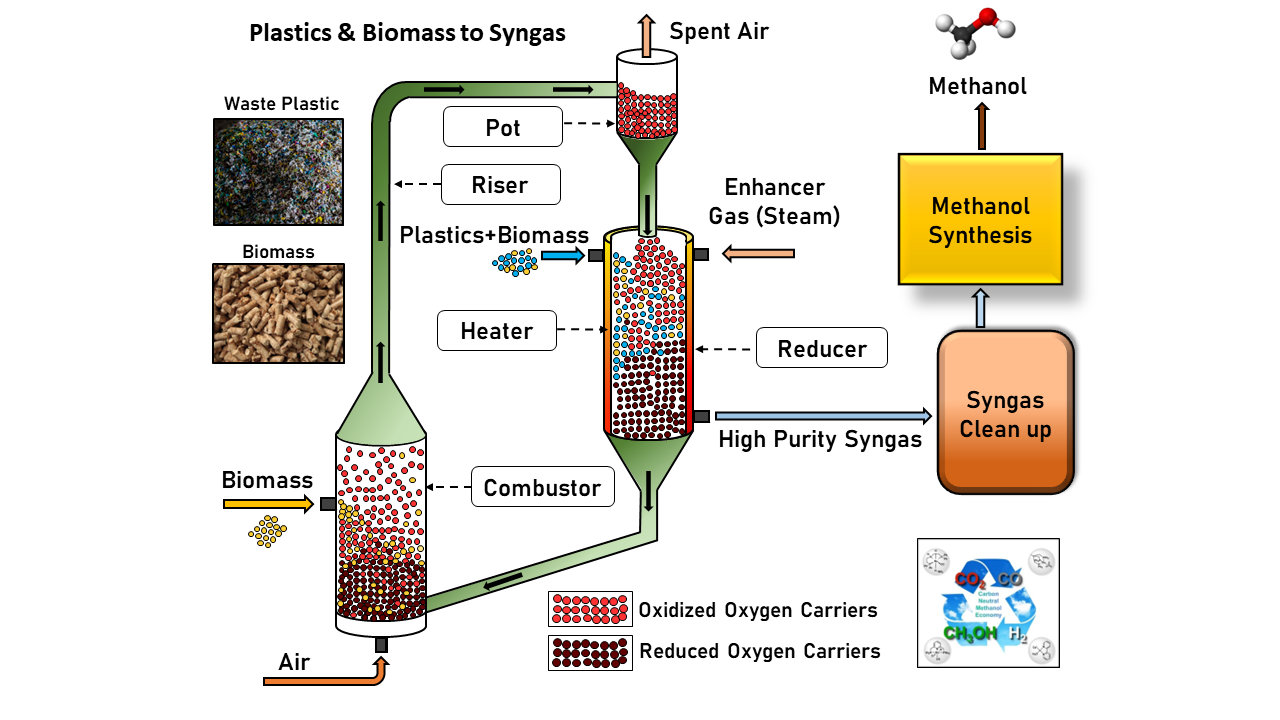
The chemical looping process is a two-reactor system that combines plastic and biomass as feedstock. The feedstock in the first reactor, a moving bed reducer, and iron-based oxygen carriers move concurrently. The steam is added along with feedstock for char gasification. The oxygen carriers donate their lattice oxygen to carbon from the feedstock during this process, forming carbon monoxide (CO) and hydrogen (H2). The result is high-purity syngas, i.e., CO+H2, which can be upgraded to valuable chemicals in downstream processing. The second reactor, a fluidized bed combustor, regenerates the oxygen carriers using air and recirculates them back to the reducer. This process eliminates the need for expensive units like tar reformers, air separation, and acid gas removers.
The gasification process is highly endothermic; therefore, several approaches have been explored to counteract this and achieve an autothermal system, i.e., no external heat is required. One of these methods involves burning biomass in the combustor to supply heat to the process, while another involves co-feeding natural gas, waste plastics, and biomass into the system. This serves two purposes: creating an autothermal system due to the high heating value of natural gas, and using natural gas as a hydrogen source to produce higher-quality syngas. In the proposed strategies, the syngas purity is higher than 90%, and H2:CO is approximately 2, which is required for downstream processing of valuable chemicals such as methanol. Compared to the existing indirect gasification of mixed plastic waste, the proposed strategies outperformed them. The indirect gasification process relies on oxygen from an air separation unit to burn natural gas, generating heat for plastic gasification while producing carbon emissions, either treated by a highly energy-intensive acid gas removal process or left untreated. This process was simulated using Aspen Plus software. The feasibility of the process was also investigated by conducting experiments on a 2.5 kW thermal bench scale unit. The coherence between the experimental and simulated data was verified, indicating that the process is feasible.