Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Meet the Candidates Poster Sessions
- Meet the Faculty and Post-Doc Candidates Poster Session
- (4oz) Enhancing Lithium-Sulfur Batteries with Nanoscale Crystalline-Amorphous Oxide

Li-S batteries, characterized by sulfur cathodes, achieve energy densities of up to 2600 Wh/kg, offering more than five times the specific capacity of Li-ion batteries. However, the practical application of Li-S batteries is hampered by the inefficient utilization of sulfur within the cathode. The discharging process of Li-S batteries involves a multi-step and multi-phase conversion from elemental sulfur to lithium sulfide (Li2S), passing through an intermediate stage of lithium polysulfides (LiPSs). The conversion of LiPSs to Li2S plays a pivotal role in the discharge process, representing 75% of the total discharge capacity; however, this conversion is sluggish because of both the high solubility of LiPS in the electrolyte and the thermodynamic unfavorability stemming from the relatively low adsorption energy of Li2S.
Consequently, significant efforts have been dedicated to enhancing the LiPS-Li2S conversion process; specifically, recognizing that this conversion encompasses the adsorption of LiPS followed by its electrochemical reduction, heterogeneous cathode substrates have been actively employed to facilitate each step. Accordingly, much research has been conducted applying cathode electrocatalysts with distinct adsorption and conversion characteristics to promote the adsorption and conversion of LiPS. Generally, on electrocatalyst surfaces with strong LiPS adsorption properties, LiPS diffusion characteristics are slow, and thus the conversion reaction is also slow. Therefore, if materials with different properties are integrated at the microdomain level, it is expected that the conversion characteristics will improve due to enhanced LiPS diffusion speed, but research in this direction has not yet been conducted.
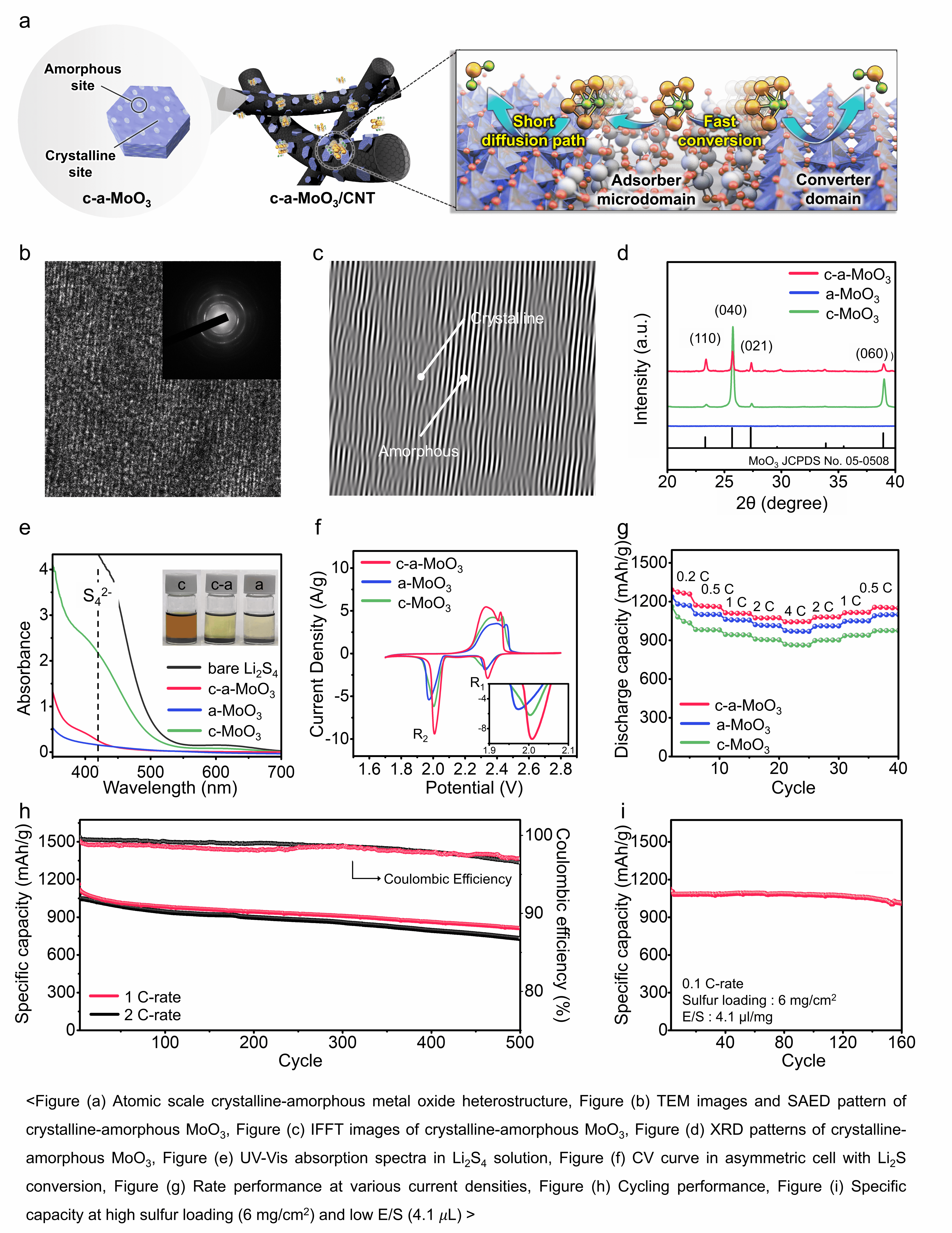
Here, we present a nanoscale oxide electrocatalyst composed of crystalline and amorphous phases. This electrocatalyst induces the adsorption of LiPS at the amorphous site and the conversion of LiPS at the crystalline site, with each domain integrated on a nanometer scale, which is expected to improve conversion due to reduced LiPS diffusion length. By applying this electrocatalyst to the cathode of a lithium-sulfur battery, we aim to achieve a lithium-sulfur battery with a capacity exceeding that of lithium-ion batteries.
Results and Discussion
We fabricated a cathode composite with a crystalline-amorphous phase metal oxide (MoO3, referred to as c-a-MoO3) on the surface of a carbon substrate. This electrocatalyst was prepared using the impregnation method, and TEM, SAED patterns, IFFT imaging, and XRD peak analysis confirmed the presence of a mixed crystalline and amorphous form. Visual adsorption tests and UV-Vis spectra measurements confirmed a high adsorption characteristic for LiPS on this cathode. Additionally, we conducted CV evaluations and observed active conversion kinetics with low polarization and high peak current during the LiPS to Li2S to S8 redox processes.
By applying this cathode, we evaluated performance at various current densities, confirming a high specific capacity of 1291 mAh/g at a 0.2 C rate and 1048 mAh/g at a 4 C rate. Furthermore, we conducted long-term evaluations at 1 C and 2 C rates over 500 cycles, confirming a capacity loss per cycle (coulombic efficiency) of 0.0713% (97%) at 1 C rate and 0.0784% (96%) at 2 C rate. For practical cell evaluation, we tested the cell under high sulfur loading (6 mg/cm2) and low E/S (4.1 μL) conditions, confirming an initial capacity of 1107 mAh/g and a specific capacity of 1011 mAh/g over 160 cycles with a low capacity decay rate of 0.05%.