2024 AIChE Annual Meeting
(4oa) Characterizing Adipocyte-Tumor Intercellular Communication through Biomaterial and Microfluidic Design
Author
With the pandemic of obesity increasing at alarming rates, understanding the role of its primary cell, the adipocyte, in governing pathologies is of utmost importance. The adipocyte functions as both an adaptive and innate immune cell and is implicated in immunologic stress. On the other hand, the metastatic cascade is interweaved with inflammatory responses. Therefore, the central aim of my lab is to comprehend how the loss of homeostasis during obesity transforms the tumor microenvironment. To investigate the interaction between the adipocyte and the cancer cell, my team will pursue the following research projects:
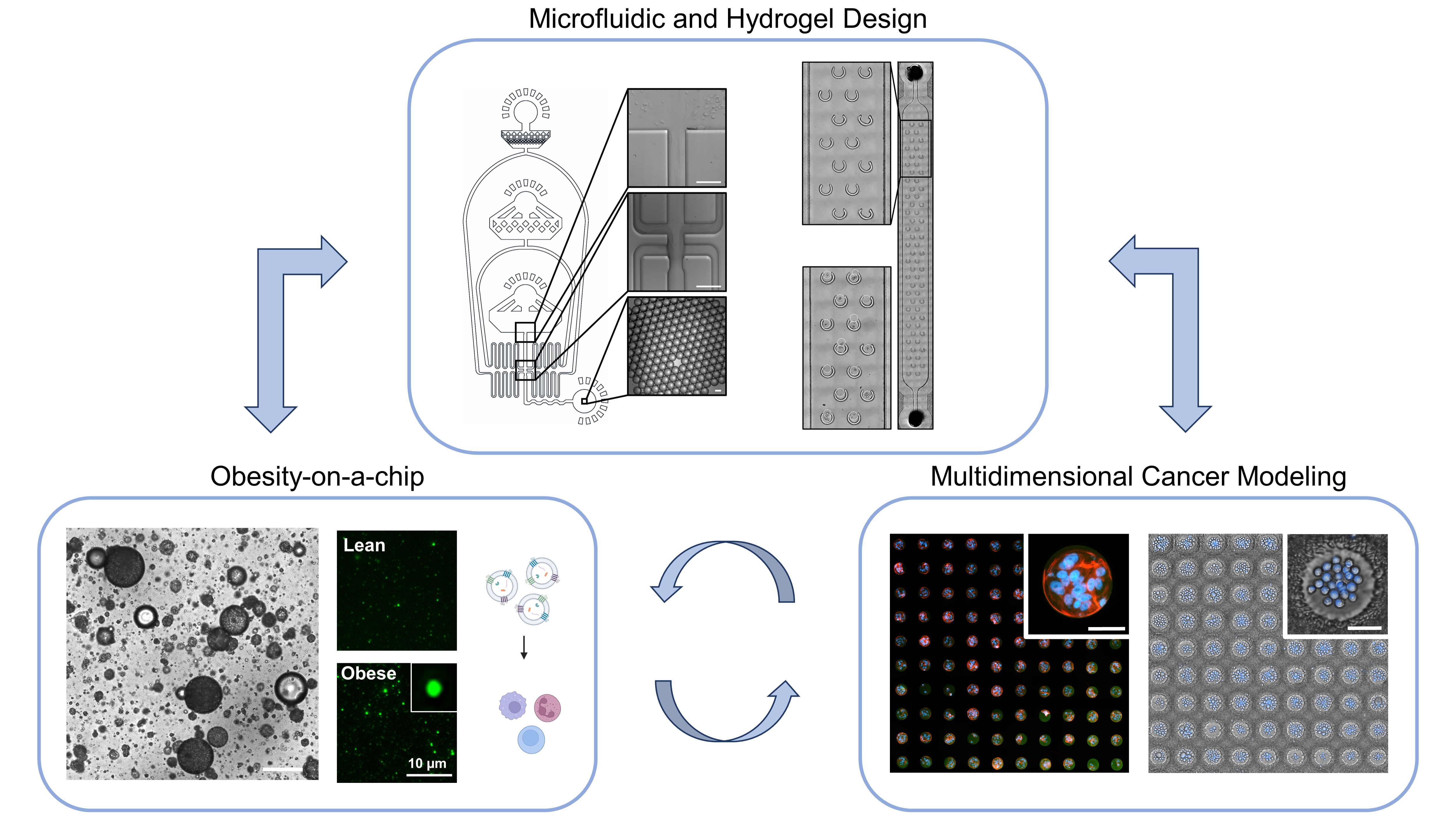
Project 1: Designing Obesity-on-a-Chip
The goal of this project is to recapitulate the morphologies of adipose tissue in people with obesity through biomaterial approaches as three-dimensional structures. Currently, the gold-standard method to investigate obesity is with animal models. However, with the United States Food and Drug Administration (FDA) Modernization Act 2.0, I believe novel in vitro models are in the spotlight of translational research.
Project 2: Model Steps of Metastatic Cascade
The goal of this project is to develop high-throughput models that simulate intravasation, circulation, and matrix remodeling through biomaterial and microfluidic design. The bioengineered assays will be coupled with high-resolution microscopy to investigate the role of chemical and mechanical sensing on proliferation, dormancy, drug resistance, migration, epithelial-to-mesenchymal transition, and immune evasion.
Project 3: Induce Intercellular Communication
Microfluidic devices will be developed to induce contact-based and non-contact-based intercellular interactions between adipocytes and cancer cells. Secretory profiles of obesity-on-a-chip, metastasis-on-a-chip, and interaction-inducing devices will be investigated, including soluble factors and extracellular vesicles (EVs). The profiles will inform independent factors that are altered with the interaction.
The success of this work will advance the fields of obesity and cancer research independently and highlight the interaction of the two. The proposed research offers various opportunities for students to learn traditional molecular biology and purification techniques and develop novel technologies, encouraging job opportunities in industry and academia. Furthermore, I have designed the research project as two independent fields to give students ownership and promote collaboration. Furthermore, the projects will require similar technologies, allowing for facile troubleshooting.
Past Experiences:
Graduate School
I conducted my doctoral training in Chemical and Biomolecular Engineering, advised by Dr. Eduardo Reátegui. My research career focused on developing single-extracellular particle technologies for the prediction of immunotherapy responses [1], systematic profiling of RNA and proteins in intact extracellular particles [2], and the orthogonal detection of single EVs via optical and non-optical modalities [3]. On the other hand, I have created large-scale arrays of cells in vitro to model intercellular interactions of non-small cell lung cancer and neutrophils [4], EV secretory profiles of invasive ductal carcinoma cells [5], and dormancy in invasive lobular carcinoma (ILC) [6]. My proudest achievement was receiving a presentation award at the International ILC Symposium as leading researchers in the field considered our work relevant to ILC patients.
Postdoctoral Training
Having been at the forefront of technology development, I focused my postdoctoral training on advancing my knowledge in medical and translational sciences. Since I believe that obesity is a major health crisis, I was admitted into a National Institutes of Health (NIH) T32 Postdoctoral Cardiometabolic Science Program aimed at fostering future leaders in the field. I am advised by Dr. Willa Hsueh, a physician-scientist, the Director of the Diabetes and Metabolism Research Center at The Ohio State University Wexner Medical Center, and the Director of the T32 Program. Currently, my work focuses on utilizing murine models to investigate the role of adipocyte-induced inflammation and EV-mediated signaling in aggravating obesity-related diseases, such as atherosclerosis and metabolic-dysfunction-associated fatty liver disease.
I am confident my Ph.D. thesis and postdoctoral training uniquely combine and give me the unique expertise necessary to conduct the proposed research as an independent researcher.
Teaching Interests:
My pedagogy for engineering education is driven by three goals: (1) acquiring the fundamental theories governing engineering practices through active learning and real-time evaluation, (2) utilizing those theories to solve both abstract and real-world problems, and (3) applying problem-solving in a collaborative context to enhance teamwork and communication as global thinkers. These are the strategies I have utilized when training undergraduate students in the lab as a mentor and in class as a teaching assistant. With my diverse background in Chemical Engineering, a minor in Applied Mathematics, and postdoctoral training in Endocrinology, I offer a unique perspective to Chemical Engineering education. I am confident in leading classes in Thermodynamics, bringing in the entropic modeling of cancer; Fluid Dynamics, bringing ideas of vascular flow; Kinetics, bringing kinetics-based equations to model micrometastatic growth; and Physical Chemistry, just because I love complex math. However, I am open and confident to teach other Chemical Engineering courses and participate in course development.
Outreach and Service:
I believe that outreach and service are equally important to academia. Since my undergraduate career, I have been a proponent of academic inclusivity, serving as a mentor, tutor, and panelist for local and national organizations, including the NextProf Pathfinder workshops. Having assumed various leadership positions in the Latinx community in graduate school, I have participated in dialogues to minimize racial biases on campus and provided recommendations to a task force. Currently, I am the community chair for LatinXinBME, where I aid in organizing events that promote community building. As a professor, I hope to involve the underrepresented community through early exposure to paid research opportunities, encourage diverse speakers in seminar series, and maintain an active presence in Latinx student organizations.
Selected References:
[1] Nguyen, L. T. H.,* Zhang, J.,* Rima, X. Y.,* Wang, X., Kwak, K. J., Okimoto, T., Amann, J., Yoon, M. J., Shukuya, T., Chiang, C. L., Waters, N., Ma, Y., Belcher, D., Li, H., Palmer, A. F., Carbone, D. P., Lee, L. J., & Reátegui, E. (2022). An immunogold single extracellular vesicular RNA and protein (AuSERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients. Journal of Extracellular Vesicles. 11(9), e12258. *Equal contribution. Featured in the NIH Director’s Blog.
[2] Zhang, J.,* Rima, X. Y.,* Wang, X.,* Nguyen, L. T., Huntoon, K., Ma, Y., Loreto Palacio, P., Nguyen, K. T., Albert, K., Duong-Thi, M. D., Walters, N., Kwak, K. J., Yoon, M. J., Li, H., Doon-Ralls, J., Hisey, C. L., Lee, D., Wang, Y., Ha, J., Scherler, K., Fallen, S., Lee, I., Palmer, A. F., Jiang, W., Magaña, S. M., Wang, K., Kim, B. Y. S., Lee, L. J., & Reátegui, E. (2023). Engineering a Tunable Micropattern-Array Assay to Sort Single Extracellular Vesicles and Particles to Detect RNA and Protein In Situ. Journal of Extracellular Vesicles. 12(11), 12369. *Equal contribution.
[3] Nguyen, K. T.,* Rima, X. Y., * Hisey, C. L., Doon-Ralls, J., Nagaraj, C. K., & Reátegui, E. (2024). Limiting Brownian Motion to Enhance Immunogold Phenotyping and Superimpose Optical and Non-Optical Single-EP Analyses. bioRxiv, 2024-02. *Equal contribution.
[4] Rima, X. Y., Walters, N., Nguyen, L. T. H., & Reátegui, E. (2020). Surface engineering within a microchannel for hydrodynamic and self-assembled cell patterning. Biomicrofluidics, 14(1), 014104. Front Cover, Featured as a Scilight, Editor’s Pick.
[5] Rima, X. Y., Zhang, J., Nguyen, L. T. H., Rajasuriyar, A., Yoon, M. J., Chiang, C. L., Walters, N., Kwak, K. J., Lee, L. J., & Reátegui, E. (2022). Microfluidic harvesting of breast cancer multicellular tumor spheroid-derived extracellular vesicles from immobilized microgels for single-vesicle analysis. Lab on a Chip. 22(13), 2502-2518. Outside Back Cover, Featured as a HOT Article, Featured as a Prime PRIMO Users' Papers for 2022.
[6] Rima, X. Y., Majumder, S., Hu, C., Li, H., Patel, D. S., Doon-Ralls, J., Nguyen, K. T., Nagaraj, C. K., Shankar, E., Stover, D. G., Zhang, X., Ramaswamy, B., & Reátegui, E. (2024). Multidimensional Bioengineering to Model Dormancy in Invasive Lobular Carcinoma. Submitted.