2024 AIChE Annual Meeting
(4iv) Design of Multi-Component Biomaterial Scaffolds for Localized Immunomodulation
Authors
The immune system as a critical regulator of homeostasis and tissue repair/regeneration: The immune system not only defends the body against pathogenic invaders but also helps maintain tissue function. Disruption of immune homeostasis severely impairs the regenerative response to an injury, and modulating the inflammatory response can unlock an otherwise inaccessible reparative sequence. Certain organs have a higher level of intrinsic regenerative capability (e.g., the liver, skin, bone) than others (the heart, central nervous system, dentin, cornea). Even for the same tissue, the intrinsic regenerative capacity declines with age. For example, the neonatal heart has a much higher regenerative ability than the adult heart. The immune system is a major contributor to the regenerative/reparative capability of an organ. For example, type 2 immunity underlies the superior regenerative capacity of the neonatal heart. Strategies that appropriately leverage the reparative immune response have broad therapeutic potential.
Objective: Guiding the course of the inflammatory response in injured tissues is a key goal of regenerative tissue engineering. Although chronic hyperinflammation after sterile injury (e.g., myocardial infarction) or pathogenic infection (e.g., viral myocarditis) directly precipitates poor clinical outcome, non-specific immunosuppression has proven to be an inadequate strategy to improve tissue healing in such scenarios. My main objective is to harness the homeostatic immunomodulatory machinery of the innate immune system using multi-component hydrogels [1] functionalized with peptides [2, 3] and drug/nanoparticle payloads to guide post-injury tissue repair while sparing distant organs from off-target effects of such interventions.
Proposed strategy: In injured tissues, a subset of stromal cells and resident macrophages undergo programmed death, and neutrophils and monocytes infiltrate the injury site from the systemic circulation. Monocytes differentiate into macrophages in situ; chemokine/cytokines released by the cells contribute to the establishment of a hyperinflammatory milieu. A subsequent resolution stage, facilitated by reparative macrophages, is accompanied by angiogenesis and fibrosis. This classic sequence of immune response can be modulated at three levels by targeting: (a) cell–matrix communication, (b) intercellular signaling, and (c) intracellular metabolism. I propose to develop a comprehensive implantable platform that directs these hierarchical processes in the innate immune system to facilitate tissue repair.
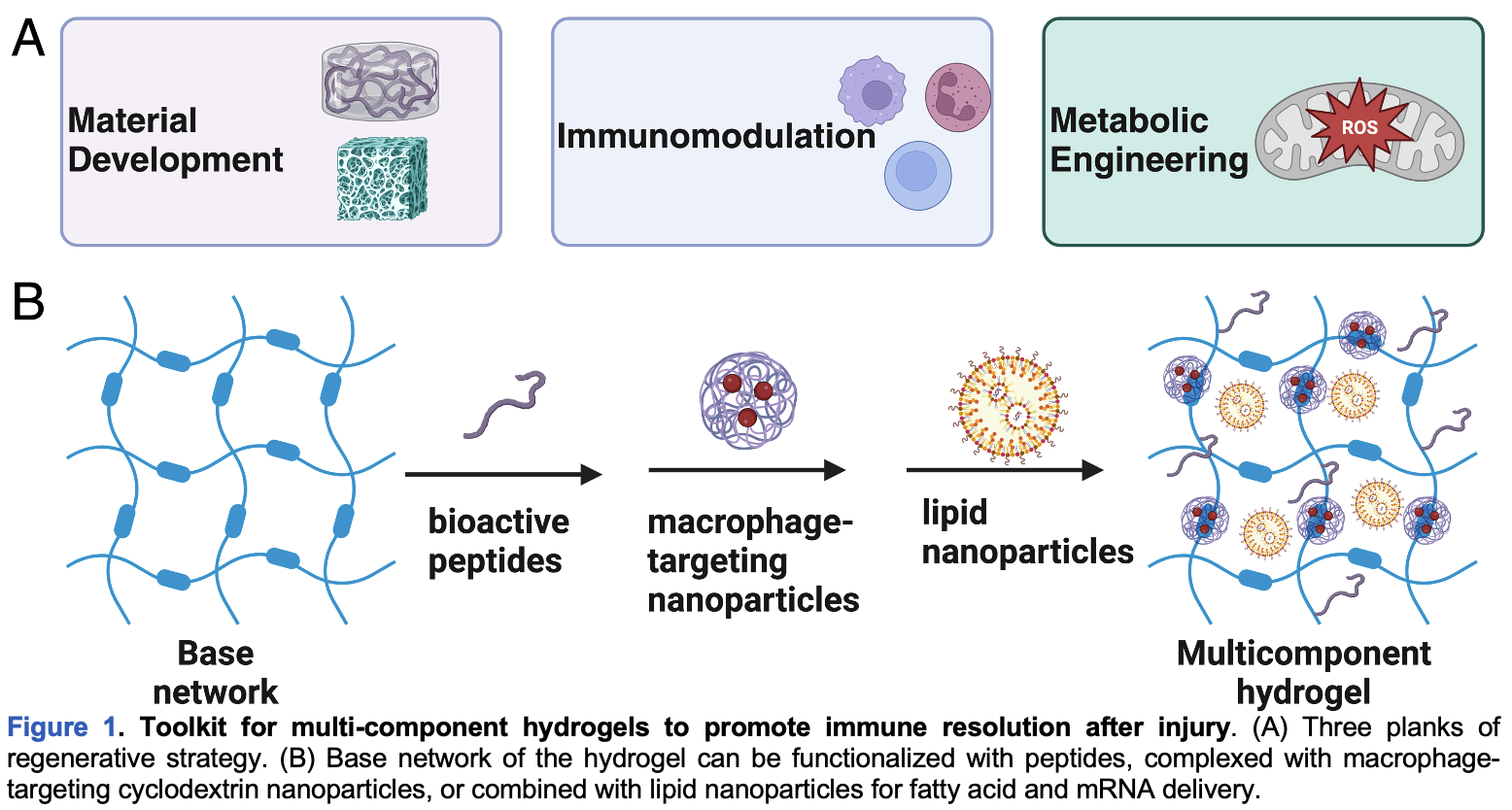
Intellectual merit: The key innovation proposed here is the development of spatiotemporally programmed multi-component constructs to program both acute inflammation and long-term immune resolution (Figure 1). Sequential immunomodulation can enable both short-term clearance of dead/apoptotic/defective cells in the injured tissue microenvironment and long-term integration of the neo-tissue with the existing tissue architecture via vasculature. Modularity of the multi-component hydrogels allows for both scaffold-based signaling aimed at cell-surface receptors and intracellular delivery of small molecules and nanoparticles (NPs). Injectability (shear responsiveness) and extracellular matrix (ECM)-mimicking nature of the hydrogel systems will further facilitate their clinical application [1, 2, 4, 5]. Systemic delivery of immunomodulatory drugs is associated with elevated risk of infection, hindering their clinical acceptance and motivating localized delivery approaches that overcome barriers to small-molecule pharmacokinetics (e.g., renal excretion, poor cell or tissue selectivity). Drug-laden hydrogels can be formulated in a syringe and injected via fine needles or meter-long catheters for tissue-specific delivery using minimally invasive procedures.
Localized delivery of drug-loaded NPs (enabled by guest–host hydrogels) in a pathological context can minimize off-target effects in distant organs [6]. Multi-component acellular hydrogels that combine scaffold-based “outside-in” signaling with delivery of mRNA-loaded NPs (“inside-out” signaling) represent the next generation of modular biomaterials for the phenotypic control of immune cells.
Functional tissue repair and regeneration requires the recruitment and integration of various cells with concomitant deposition of non-pathologic extracellular matrix components into multilayered, segregated, and hierarchical functional niches, while maintaining the material properties required for tissue function. Acellular biomimetic hydrogels (with patterned peptide-based signals and immunomodulatory payloads) developed here will be useful to control the immune consequences of tissue damage and promote implant–tissue integration. A core idea for the research proposal is that effective tissue repair and regeneration requires a permissive niche, metabolic fuel, and a signal. Dysregulation of this response may promote or support tumorigenesis, fibrosis, and other pathologies.
Platform technology: Biomaterial scaffolds or hydrogels mimic the ECM and directly interact with stromal and immune cells. Specifically, hydrogels are excellent platforms for the localized delivery of bioactive factors that can mediate cell–cell interactions. To modulate the innate immune response after tissue injury, I will develop multi-component hydrogels that spatiotemporally program the microenvironment and guide the vascularization and integration of the neo-tissue (Figure 1). Success of my strategy rests upon the combination of sequential drug delivery from guest–host hydrogels with scaffold-based signaling afforded by functional peptides encoded in the network. I will test the idea that implantable biomaterials can control the innate immune sequelae requisite for tissue repair and regeneration.
My previous research has established that the hydrogels with distinct combinations of material and biofunctional properties can be used to promote tissue regeneration [1, 2, 4, 5]. Here, I propose that bioactive peptides can be grafted directly, via covalent linkages afforded by bioconjugate chemistry, onto guest–host hydrogels to retain the biofunctionality of the former while taking advantage of the drug-delivery capability of the latter. Bioactive peptide domains can assist integration of biomaterials with the host tissue [1, 2, 4, 5], via scaffold-based signaling afforded by their interaction with cell-surface receptors. The “base network” of the hydrogel can not only be functionalized with bioactive peptides via pendant modification but also be loaded with immunomodulatory drugs and NPs (Figure 1). Crucially, the platform allows fast release of hydrophilic immunostimulatory drugs from the hydrogel and slow release of hydrophobic immunosuppressive payloads from a lipophilic binding pocket within NPs, allowing a sequence where acute inflammation is followed by long-term immuno-resolution. The core premise of the multi-component construct is that the sequence of immunomodulation is as important as localized tuning of macrophage phenotypes for ensuring tissue repair and regeneration after injury.
Broader impacts: As macrophages are key mediators of tissue repair and regeneration, the outcomes of the proposed research program are widely applicable toward immuno-regenerative medicine. Moreover, the project will yield a targeted biomaterial system to ameliorate macrophage-mediated inflammation that is applicable in various pathological contexts, such as obesity, cancer, heart failure, and chronic kidney disease.
Teaching Interests:
Cultivating a Sense of Wonder and Delight: In my experience, focusing on the underlying principles of science and emphasizing the power of chemical engineering to solve real-world problems engages the interest of students. I have a proven track record in teaching at the undergraduate level (in chemistry & biomolecular sciences). I have developed the pedagogical platform through training 13 undergrads and 7 grad students during my research career, and by teaching >60 undergraduate students as an instructor (see: https://digitalcommons.njit.edu/chem-syllabi/141). My doctoral work in Chemistry and postdoctoral research in Biomedical Engineering has prepared me to discuss the intersection of chemistry and engineering with students.
Encouraging Peer-Teaching and Group Learning: The most common feature of the effective teachers I’ve had is that they tended to encourage the students to learn the materials by themselves and by discussing the ideas with their peers. I would encourage students to collaborate among themselves on group projects. Bringing research into the classroom (active in-class article discussions) will reinforce real-world applications in a student (rather than instructor)-led fashion.
Facilitating Interdisciplinary Synthesis of Ideas: At the frontiers of research, interfaces between subject matters tend to be fuzzy. I would strive to cultivate an appreciation in my students that often natural sciences tend to provide different perspectives on the same underlying natural phenomena: such as free energy, entropy, nucleation phenomena, fluctuation–dissipation, and charge complementarity. Topics such as thermodynamics and biochemistry could help the students become better chemical engineers!
Focusing on the Writing and Presentation of Students: Encouraging students to write long-form would encourage them to seek out primary literature and synthesize distinct ideas into a coherent perspective. I believe, as future scientists and engineers, they will be responsible for effective communication, research and problem solving. Key components of my classes will include group work, presentations, problem-based learning, engineering design, idea development and research proposals, in addition to canonical problems sets, midterms and finals.
Developing an Evidence-based Curriculum: Randomized controlled studies on teaching effectiveness and instructor evaluations will guide my design of curricula. I aim to be mindful of the socioeconomic differences and inequities. Finally, I would advise and direct undergraduate students to obtain hands-on lab research skills that fit their research interests, which will help them get to graduate schools and improve their critical thinking skills. My broad educational background (in chemistry and engineering) has prepared me to teach a wide range of courses in this field.
References:
[1] Z. Siddiqui, B. Sarkar, et al. Chem Eng J 422 (2021) 130145.
[2] Z. Siddiqui, B. Sarkar, et al. Acta Biomater 126 (2021) 109-118.
[3] B. Sarkar et al. J Am Chem Soc 136(41) (2014) 14417-24.
[4] B. Sarkar et al. Biomacromolecules 19(9) (2018) 3597-3611.
[5] B. Sarkar et al. Chem Eng J 408 (2021) 127295.