2024 AIChE Annual Meeting
(4ir) Developing Polymer-Based Electrolyte for Next-Generation Rechargeable Battery
Author
Demand for high-performance rechargeable batteries continues to grow as electric vehicle production increases. The energy density of lithium-ion batteries, used in this application, has not significantly improved in the past decade. Electrodes such as silicon and lithium metal have the potential to increase energy density, but electrolytes that are stable against these electrodes are not yet been identified. Polymer electrolytes have the potential to stabilize these electrodes. Lithium salts dissolved in poly(ethylene oxide) (PEO) were first hypothesized for battery electrolyte applications in 1979 by M. Armand. It is not clear if any of the polymer electrolytes developed in subsequent years is better for battery applications.
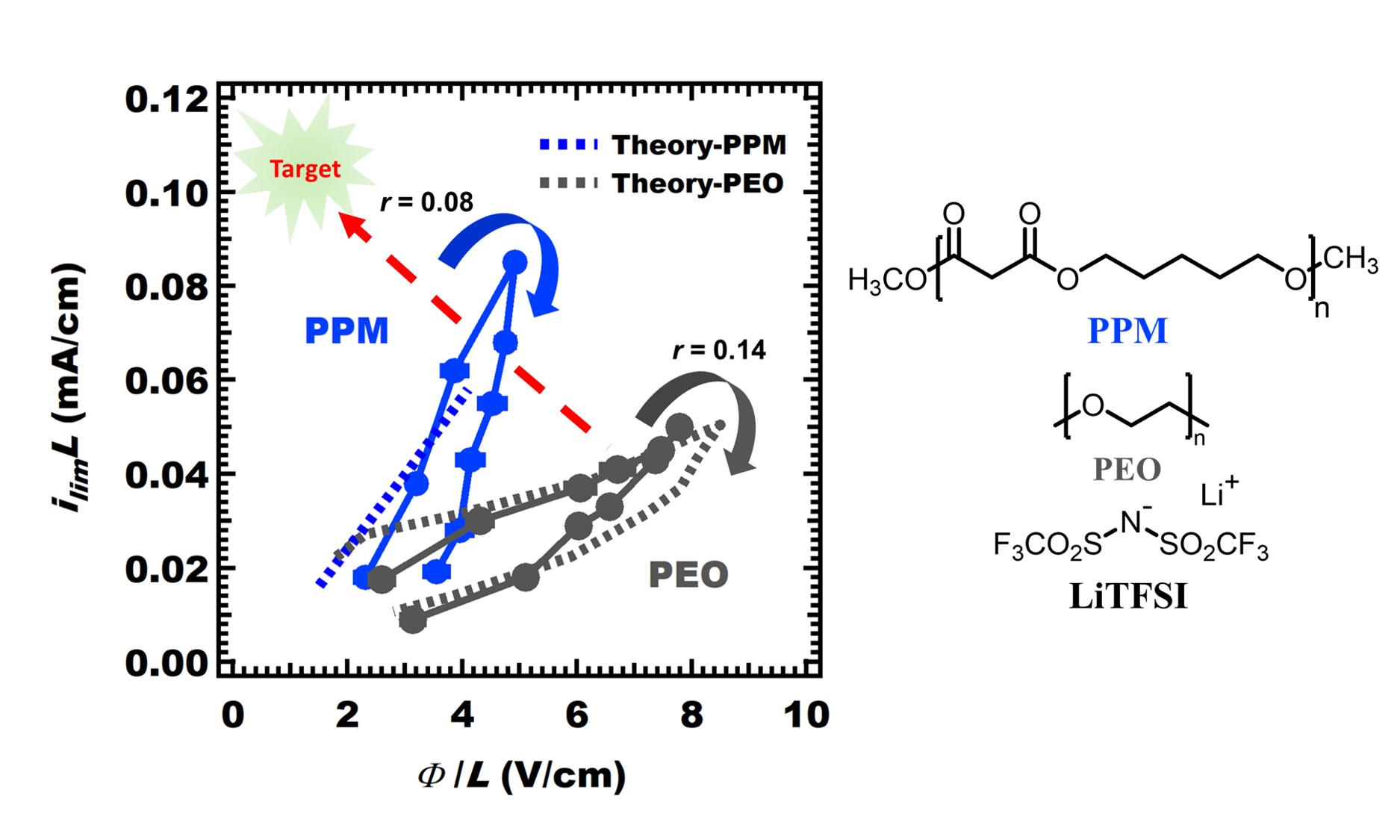
For practical applications, electrolytes must support large currents. The limiting current density is the maximum current density which can be stably applied across an electrolyte. Salt concentration gradients develop when current passes through an electrolyte, resulting in high concentration near the positive electrode and low concentration near the negative electrode. At the limiting current, either the concentration at the negative electrode approaches zero, or the concentration at the positive electrode approaches the solubility limit. The limiting current is a convenient metric for comparing different electrolytes. To my knowledge, there is no polymer electrolyte present in the published literatures that exhibits a higher limiting current than PEO. Another metric for comparing different electrolytes is the potential drop needed to sustain the limiting current. An ideal electrolyte will exhibit the lowest potential drop and the highest limiting current.
The limiting current and potential drop through an electrolyte can be predicted by Newman’s concentrated solution theory. Applying this theory requires knowledge of four concentration-dependent parameters. In a previous study, it was shown that the limiting current can be predicted using conductivity, κ, salt diffusion coefficient, salt diffusion coefficient, D, current fraction measured in Bruce-Vincent experiment, ρ+, and the open circuit potential of concentration cells, U.
I measured the values for poly(pentyl malonate) (PPM) electrolyte. The predicted values of limiting current and potential drop are in good agreement with experimental measurements, which were conducted in symmetric cells using lithium-indium alloy electrodes. The maximum limiting current value of PPM/LiTFSI is about 1.7 times higher than that of PEO/LiTFSI, and the potential drop at the maximum limiting current density through PPM/LiTFSI electrolyte is about 1.6 times smaller than that of PEO/LiTFSI. I present a new plot for comparing the performance of different electrolytes.
I plan to broaden my research scope using a stable alloy electrode with a polymer electrolyte platform, transitioning from metal electrode symmetric cells to full cells. After incorporating a cathode electrode, my aim is to investigate the relationship between ion transport through polymer electrolytes and battery performance metrics such as charge/discharge rates and cycling stability. The outcomes of this project are expected to inform the design of full cell batteries utilizing polymer electrolytes.
I plan to broaden my research scope using a stable alloy electrode with a polymer electrolyte platform, transitioning from metal electrode symmetric cells to full cells. After incorporating a cathode electrode, my aim is to investigate the relationship between ion transport through polymer electrolytes and battery performance metrics such as charge/discharge rates and cycling stability. The outcomes of this project are expected to inform the design of full cell batteries utilizing polymer electrolytes.
Furthermore, my ongoing commitment involves addressing inherent challenges in polymer electrolyte systems, particularly poor ion transport at room temperature. Following my post-doc research, I have focused on enhancing ion transport through several approaches:
Firstly, leveraging my polymer synthesis skills honed during my PhD in South Korea, I synthesized not only PPM, but also single-ion conductor polymers. I developed multiple polymer blends incorporating PEO and explored their phase behavior using small-angle neutron scattering (SANS) experiments. Remarkably, PPM/PEO blend electrolytes show a completely miscible polymer blend electrolyte regardless of the addition of lithium salt This achievement represents an initial stride towards enhancing ion transport in polymer electrolytes through polymer blending.
Secondly, in collaboration with Prof. Geffory Coates from Cornell University, we innovated several novel types of polymer electrolytes. These included designs incorporating random copolymers and silaketal functional groups, which exhibited superior ion transport properties compared to PEO.
Lastly, I have embarked on fabricating polymer-inorganic conductor composite electrolytes. Unlike previous approaches, my current strategy involves consideration of the size scale between polymer chains and inorganic conductor nanoparticles. While conclusive evidence on the optimal approach to improving ion transport through polymer electrolytes remains elusive, not only find the optimal full cell configuration, these experiments are indispensable steps towards realizing polymer-based electrolytes for next-generation rechargeable batteries.
Teaching Interests
Building on my research expertise in energy storage challenges, ion transport phenomena, and polymer engineering, including synthesis and polymer physics analysis, I am enthusiastic about teaching foundational courses. These would encompass general chemistry, transport phenomena, and general polymer chemistry/physics, as well as physical chemistry. Moreover, I am particularly eager to instruct advanced courses that delve into polymer engineering tailored for energy storage applications like supercapacitors and batteries. These courses align seamlessly with my research focus and are directly pertinent to ongoing advancements in chemical engineering.
My teaching priority emphasizes imparting to students the importance of collaboration. In an era where technologies like AI are advancing rapidly, the traditional emphasis on broad but not necessarily deep knowledge in specific subjects is evolving. It is crucial for students to acquire deep understanding in their chosen subjects while also developing collaborative skills.
As AI and automation increasingly handle routine tasks, human roles are shifting towards tasks that require creativity, critical thinking, and complex problem-solving—areas where collaboration across disciplines and specialties becomes essential. Therefore, my goal is to guide students in cultivating in-depth expertise in their fields while also encouraging them to collaborate effectively across diverse domains. This approach prepares them to thrive in a future where interdisciplinary teamwork and specialized knowledge are integral to innovation and success.
Finally, I aspire to be a supportive mentor for international students, irrespective of their citizenship. Diversity encompasses not only race but also the various nationalities of students who come from different parts of the world. Being an out-of-state faculty myself, I understand the challenges of adapting to a new environment. I can provide valuable advice on settling down and adjusting to life in the United States.