2024 AIChE Annual Meeting
(4ga) Progressing Towards a Sustainable Future with Computational Research: Advancing Energy Storage to Waste Management
Author
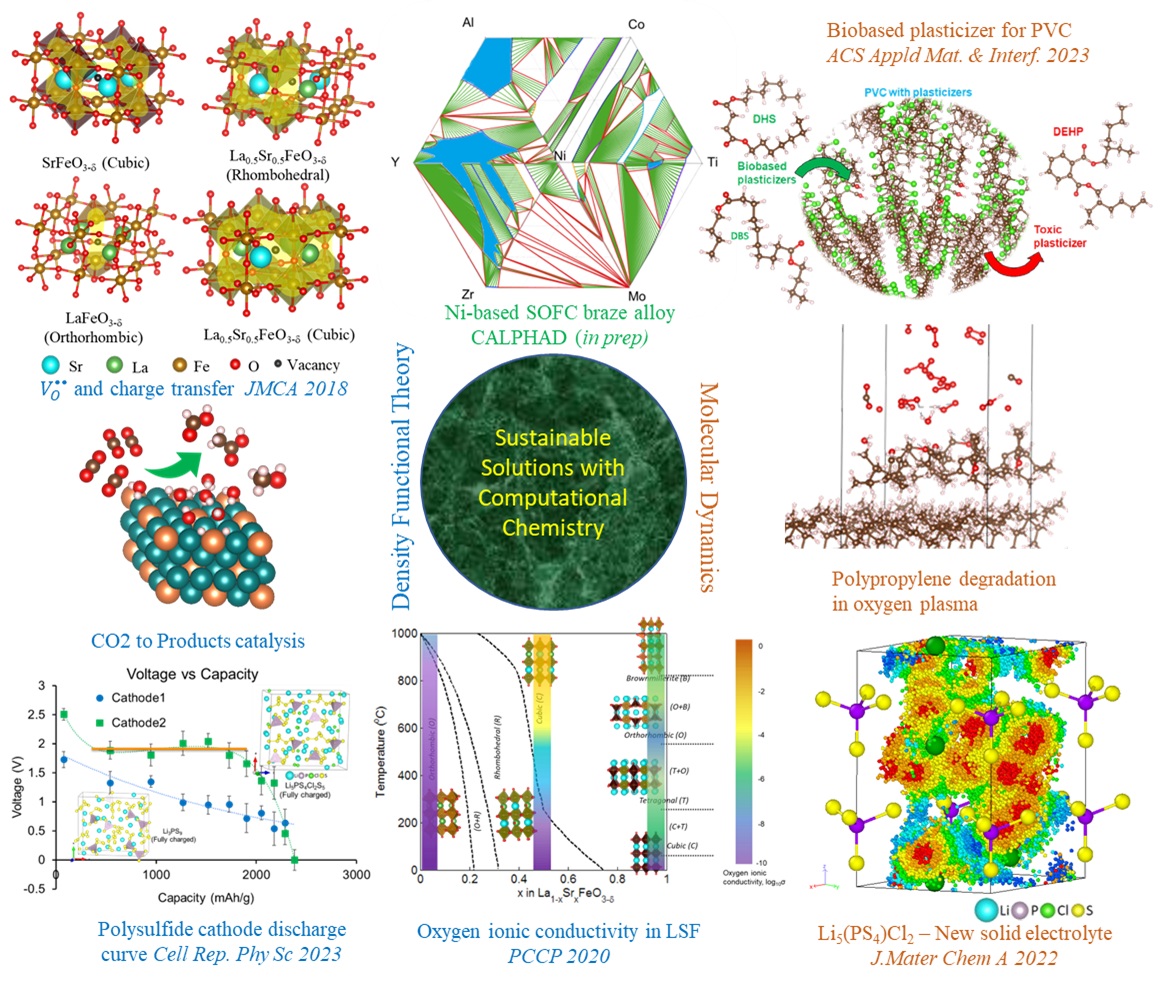
The quest for a sustainable future is a multifaceted endeavor, and computational research serves as a critical tool in this journey, interconnecting various domains from energy storage to waste management. Starting with energy storage, computational studies are instrumental in the development of solid-state batteries, a safer and more energy-dense alternative to traditional lithium-ion batteries. These studies expedite the discovery of new solid electrolytes and enhance our understanding of ion transport mechanisms. The same computational prowess also extends to the field of carbon capture and utilization, where it aids in designing catalysts that can efficiently convert CO2 into valuable products, thereby promoting a circular carbon economy. This concept of upcycling is also applicable in the realm of plastic waste management. Computational tools can identify efficient chemical pathways to transform waste plastics into valuable chemicals, contributing to environmental conservation. Lastly, these tools also find application in wastewater treatment, where computational models can optimize process parameters and predict system performance, ensuring clean water availability. Thus, through these interconnected applications, computational research is driving us towards a more sustainable future.
Solid State Electrolyte - For a sustainable future with renewable energy productions, we will need efficient energy storage devices. From electric vehicles to grid storage - solid-state batteries are favored over traditional Li-ion batteries due to their superior thermochemical stability, enhanced safety, and extended cycle life. The effectiveness of solid-state batteries is largely dependent on the stability of the electrode-electrolyte interface and the rapid transport of Li-ions in the electrolyte. Among various electrolyte candidates from the polysulfide, oxide, argyrodite, and perovskite families, Li6PS5Cl stands out due to its superionic conductivity of lithium at room temperature and broad electrochemical stability window. We have recently discovered a new composition from the polysulfide family, Li5PS4Cl2, which exhibits highest solid-state Li-ionic conductivity at room temperature, approximately 24 mS/cm. We have also examined the thermochemical stability of these electrolytes with an S-based cathodes. We studied the interfacial stability of the solid electrolyte with S-based cathode and Li-anode using Reactive Molecular Dynamics. Reax force field parameters were developed using DFT studies on small molecular systems. The discharge process was simulated by incrementally adding Li to the fully charged state (Li3PS9), and the discharged structure was obtained (Li12PS9) and was well compared with the experimental discharge curve, as shown in our recent publication.
Water purification - The development of computational methods to extract valuable metals from wastewater is a burgeoning field of research, contributing significantly to resource recovery and environmental protection. One such example is the extraction of selenium, a valuable and essential trace element, from wastewater. Selenium often exists in wastewater as selenate or selenite ions. Computational methods, such as Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations, can be used to design and optimize the extraction process. For instance, DFT can predict the binding energies and adsorption sites of selenate/selenite ions on various adsorbents, guiding the selection of materials for selenium extraction. MD simulations can provide a dynamic picture of the adsorption process, offering insights into how the ions interact with the adsorbents and how this is influenced by various factors such as pH and temperature. By leveraging these computational tools, we can enhance the efficiency of selenium extraction from wastewater, turning waste into a valuable resource. This not only contributes to resource conservation but also helps in mitigating the environmental impact of wastewater discharge.
Polymer upcycling - Polymers which are most difficult to degrade are polyethylene and polypropylene. In our recent study we have reported that, to degrade PE/PP at a lower temperature, oxygen has to enter the polymeric chain. This leads to the formation of carbonyl groups and the breaking of the long hydrocarbon chains into smaller molecules that can be a feedstock for various hydrocarbon-based products. The first and most important step of this process, the natural oxidation of PE/PP polymer, usually depends on abiotic factors like light or temperature. It takes years for the long polymeric molecules to break down in natural conditions, and then bacteria or fungi can take over the degradation. This is the current framework of biodegradation research. Based on this framework, some bacterial and fungal strains have been found to degrade PE to some degree. But in most cases, they need a harsh pre-treatment of PE (heating, UV light, etc.) that speeds up the oxygen incorporation into the polymer, making the abiotic oxidation the main obstacle of the reaction. In the last ten years, a few microorganisms have been reported to act on untreated PE, but they need much more time to do so than with pre-oxidized PE. To breakdown PE, PP or PET with microorganisms is going to require a huge land mass and really long-time scale for the process. In future I plan to develop a framework with my students to computationally design inorganic catalyst by biomimicing the enzymatic degradation of PE/PP.
CO2 reduction - Catalytic conversion of carbon dioxide (CO2) into valuable products such as methane (CH4), acetate, and alcohol is a promising strategy for mitigating climate change and achieving a circular carbon economy. This process involves the use of catalysts to facilitate the transformation of CO2, a greenhouse gas, into useful chemicals. For instance, the conversion of CO2 to CH4, also known as methanation, can be catalyzed by composite metals like platinum and titanium as shown in our recent work. This reaction not only sequesters CO2 but also produces a valuable fuel. Similarly, the electrochemical reduction of CO2 can yield acetate and alcohol, which are important feedstocks in the chemical industry. The choice of catalyst and the operating conditions can influence the selectivity towards these products. Advances in catalyst design and process optimization, guided by computational studies, can enhance the efficiency and selectivity of these conversions, making them more viable for industrial application. Thus, catalytic CO2 conversion represents a key technology for sustainable development. My group will continue to work in this area with development of machine learning force fields for faster screening of new catalytic surfaces.