2024 AIChE Annual Meeting
(471g) Engineered Orthogonal Growth Factor Ligand/Receptor Pair for Safe and Effective Bone Regeneration
Author
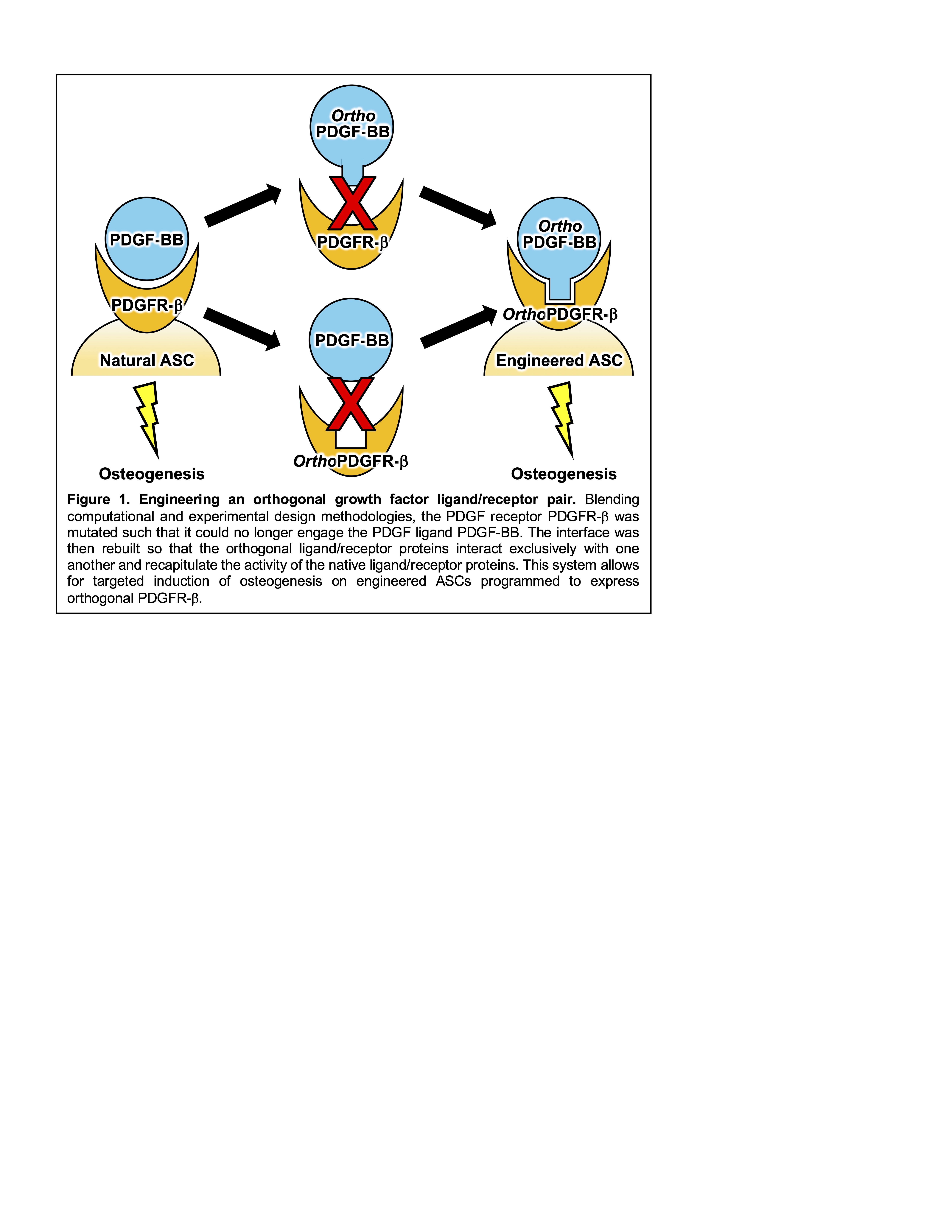
Most bone tissue engineering approaches employ marrow-derived mesenchymal stem cells (MSCs) as progenitors, and multiple clinical trials using MSC-containing scaffolds are underway; however, MSCs are of very low abundance (<0.01% of nucleated cells in the bone marrow). Adipose-derived stem cells (ASCs), which comprise >1% of nucleated cells in the stromal vascular fraction of adipose tissue, can also differentiate into the three classical mesenchymal lineages of bone, fat, and cartilage, presenting a promising alternative. It was recently shown that the platelet-derived growth factor (PDGF) ligand PDGF-BB induces robust osteogenic responses in ASCs, motivating the incorporation of this growth factor into ASC-based bone tissue engineering regimens. PDGF-BB signals through activation of its cognate receptor (PDGFR-β) to mediate a range of physiological processes including bone repair and the natural ligand is FDA approved for localized treatment of periodontal defects and diabetic ulcers. However, the powerful pleiotropic effects of PDGF-BB complicate its use in bone repair scaffolds, as this protein has been implicated in the onset and pathogenesis of diseases such as cancer, vascular disorders, and fibrotic disorders. Thus, the design of a selective version of PDGF-BB that exclusively stimulates osteogenic responses in transplanted ASCs would represent a transformative advance for the field of bone tissue engineering.
To overcome the limitations for PDGF therapy in bone regeneration, we designed a novel molecular engineered ligand/receptor pair for targeted promotion of osteogenesis. To achieve this, we devised a a hybrid computational/experimental design workflow to generate an “orthogonal” pair of proteins that are genetically mutated to interact exclusively with one another, and not with any endogenous proteins (Figure 1). Specifically, we synthesized protein structure prediction algorithms with yeast surface display-based directed evolution technologies to engineer an orthogonal PDGFR-β receptor and evolve a complementary orthogonal PDGF-BB ligand. We implemented binding studies using biolayer interferometry to establish the binding orthogonality of our ligand/receptor pair. We then demonstrated functional orthogonality by transducing our orthogonal PDGFR-β into ASCs derived from discarded tissue obtained from liposuction procedures, and establishing that orthogonal but not wild type PDGF-BB led to activation of PDGF signaling pathways and consequent osteogenic responses. Our orthogonal ligand/receptor system is currently being integrated into a tissue engineering workflow for testing in a murine critically-sized calvarial defect model. In this system, transplanted ASCs are engineered to express the orthogonal PDGFR-β receptor, embedded in a biomaterial scaffold that is implanted into mice, and then stimulated in vivo via systemic delivery of orthogonal PDGF-BB to demonstrate the potential for our strategy to mediate safe and effective bone repair. Overall, our innovative molecular engineering approach will allow, for the first time, targeted activation of tissue regeneration pathways, realizing the vast potential of growth factor therapy for regenerative medicine.