2024 AIChE Annual Meeting
(444d) Hydrated Eutectic Electrolytes for High Performance Rechargeable Zinc Batteries
Authors
Olumide Oladoyin, The University of New Mexico
Hao Nguyen, University of New Mexico
Shuya Wei, University of New Mexico
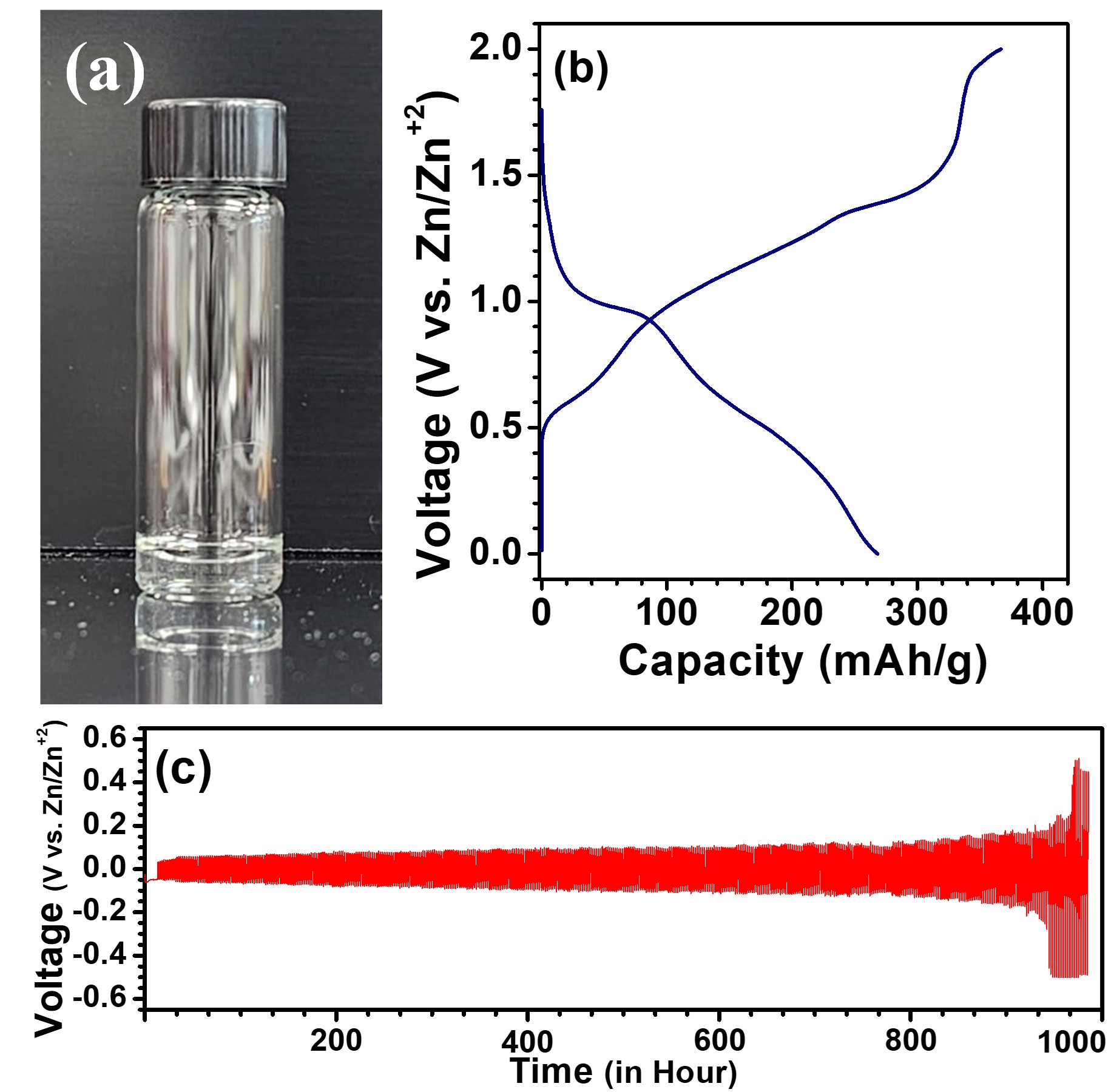
Zinc has attracted significant interest as a promising anode material in aqueous rechargeable batteries, offering advantages such as high volumetric capacity (5857 mAhcm-3 vs 2042 mAhcm-3 for Li), low cost (2.4 USD.kg-1 vs. 19.2 USD.kg-1 for Li), and multiple electrons transfer capability (2e- vs. 1e- for Li). However, the intractable side reactions at the negative Zn anode and parasitic hydrogen evolution in the aqueous media hamper the technology unattainable for practical evaluations. Here, we present a hydrated eutectic electrolyte by combining hydrated Zn salt (ZnSO4.7H2O) with triethylamine hydrochloride. Eutectic formation between the two salts is attributed to Lewis acid-base interactions, facilitated by substantial charge delocalization exhibited by the SO4- anions, serving as a strong Lewis bases. The bipolar nature of H2O readily interacts with the Zn+2, forming an ion-conducting coordination complex, thereby suppressing the hydrogen evolution reaction (HER). The electrolyte offers a high electrochemical stability window (2.45V vs. Zn/Zn+2), wide liquid range suitable (Tliquid ³-20 oC), low vapor-pressure, environment insensitivity and the symmetrical Zn||Zn cell demonstrates excellent stability for >1000 hours at 0.1 mA/cm2. When paired with V2O5, Zn exhibits a high discharge capacity of 270 mAh/g, and promising cyclability for >600 cycles. These results highlight its potential as an alternative to aqueous electrolytes for grid-level storage units.