2024 AIChE Annual Meeting
(441b) Development of Phosphate-Selective Sensors Based on Macrocyclic Ionophore-Doped Membranes
Authors
Ion-selective electrodes provide the advantages of low cost, miniaturization, and portability, thus enabling the development of more suitable and practical methods for analyzing phosphate levels in the environment. In this study, two PVC-based ion-selective flexible sensors were developed by inkjet printing technique for sensing trace amounts of monohydrogen phosphate and dihydrogen phosphate ions in water.
Conductive nanoparticle ink with the concentration of 40 wt.% were printed on a flexible polyethylene terephthalate (PET) sheet with a thickness of 140 μm, forming reference and working electrodes (0.2 cm2), respectively. The printing process was conducted using Fujifilm Dimatix 2850 drop-on-demand printer with a 10 pL cartridge. A nozzle temperature of 25 ◦C was used to generate stable droplets. The PET substrate temperature was set at 60 ◦C. Silver ink was printed on the electrode areas with a drop spacing of 10 μm for 5 layers, employing the water waveform, firing voltage from 23 to 30 V, jetting frequency of 5-30 kHz and cured at 140 ◦C for 30 min. The resulting electrode resistivity was 86 µΩ-cm.
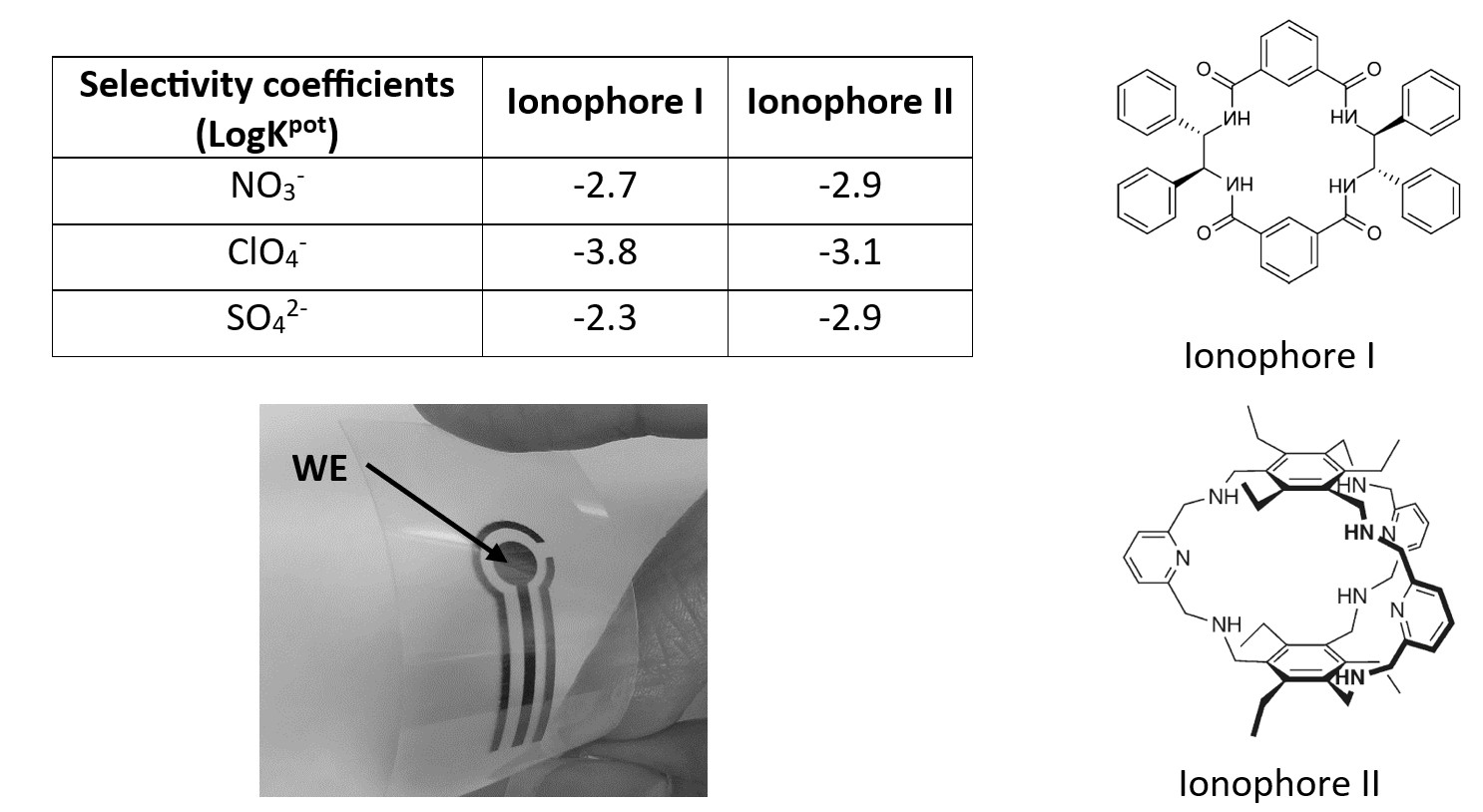
Phosphate-selective membrane cocktails composed of high-molecular-weight polyvinyl chloride, PVC (33 wt.%) as the polymeric matrix, 2-nitrophenyl octyl ether, o-NPOE (63.5 and 62.6 wt.%) as the as plasticizing solvent mediator, ionophores (50 mmol/kg membrane), and hexadecyl trimethylammonium bromide (HTAB) as the anion exchanger (10 mmol/kg membrane) were prepared by dissolving in Tetrahydrofuran and investigated as H2PO4- and HPO42- selective sensors. Ionophores were macrocyclic polyamide and polyamine compounds containing protonated nitrogen. The sensing layers were prepared by drop coating of each membrane cocktail onto the printed working electrode (WE). Potentiometric measurements were carried out at a constant temperature of 20 °C and pH of 7.4.
Potentiometric results showed that ionophore-based membrane electrodes exhibited a good sensitivity to aqueous solutions of the monohydrogen and dihydrogen orthophosphate species within the concentration range pC=2-5, with near-Nernstian response slopes of −28.3 and −50 mV per activity decade for ionophores I and II, respectively, with a response time of less than 60 s. Both sensors exhibited good reproducibility (SD<±3 mV). Selectivity coefficient values for orthophosphate ions were determined using the separate solution method and revealed negligible interference from anions, including sulfate, nitrate, and perchlorate.