2024 AIChE Annual Meeting
(41g) Bi-Philic Mesh Technology: An Electrodialysis Parallel for Next-Gen CO2 Sequestration
Author
Introduction
The menace of global warming, accentuated by man-made emissions of CO2, poses a multifaceted challenge that affects not only the macroscopic climate of our planet but also the air quality and public health within our cities. This problem is further exacerbated by traditional industrial practices and urban planning strategies, which have inadvertently contributed to the persistence and escalation of these issues. The relocation of industrial facilities away from population centers, while intuitive, merely obscures rather than resolves the core problem, as the resultant increase in transportation emissions offsets potential benefits. This presentation unfolds the narrative of a pioneering solution developed through a collaboration between our group and Lehigh University, aimed at redefining the approach to carbon capture and sequestration. This novel strategy leverages waste-heat recovery in industrial and residential settings to enable at-the-source carbon capture, presenting an energy-efficient solution that harmonizes with the spatial constraints of urban environments. The hot liquid reset the absorption resin and transport the CO2 to compressed storage sites. By adopting this strategy, we envision the development of cleaner industrial facilities that can be situated closer to urban centers, thereby reducing logistical inefficiencies and contributing to a more sustainable infrastructure.
From Problem to Innovation
The central challenge to achieving this goal is found at the juncture of absorption (carbon capture) and regeneration (long-term storage) within the process. The heated liquid carries substantial thermal potential, leading to the release of large amounts of CO2 all at once. It is crucial that all the released gas be promptly isolated and captured from the liquid for storage; otherwise, leakage and backlog could disrupt operations. To put this into perspective, even a relatively small factory can emit 1 ton of CO2 in a single day. Without timely separation, containing this volume of CO2 would require a storage cube equivalent to the size of a three-story building.
The menace of global warming, accentuated by man-made emissions of CO2, poses a multifaceted challenge that affects not only the macroscopic climate of our planet but also the air quality and public health within our cities. This problem is further exacerbated by traditional industrial practices and urban planning strategies, which have inadvertently contributed to the persistence and escalation of these issues. The relocation of industrial facilities away from population centers, while intuitive, merely obscures rather than resolves the core problem, as the resultant increase in transportation emissions offsets potential benefits. This presentation unfolds the narrative of a pioneering solution developed through a collaboration between our group and Lehigh University, aimed at redefining the approach to carbon capture and sequestration. This novel strategy leverages waste-heat recovery in industrial and residential settings to enable at-the-source carbon capture, presenting an energy-efficient solution that harmonizes with the spatial constraints of urban environments. The hot liquid reset the absorption resin and transport the CO2 to compressed storage sites. By adopting this strategy, we envision the development of cleaner industrial facilities that can be situated closer to urban centers, thereby reducing logistical inefficiencies and contributing to a more sustainable infrastructure.
From Problem to Innovation
The central challenge to achieving this goal is found at the juncture of absorption (carbon capture) and regeneration (long-term storage) within the process. The heated liquid carries substantial thermal potential, leading to the release of large amounts of CO2 all at once. It is crucial that all the released gas be promptly isolated and captured from the liquid for storage; otherwise, leakage and backlog could disrupt operations. To put this into perspective, even a relatively small factory can emit 1 ton of CO2 in a single day. Without timely separation, containing this volume of CO2 would require a storage cube equivalent to the size of a three-story building.
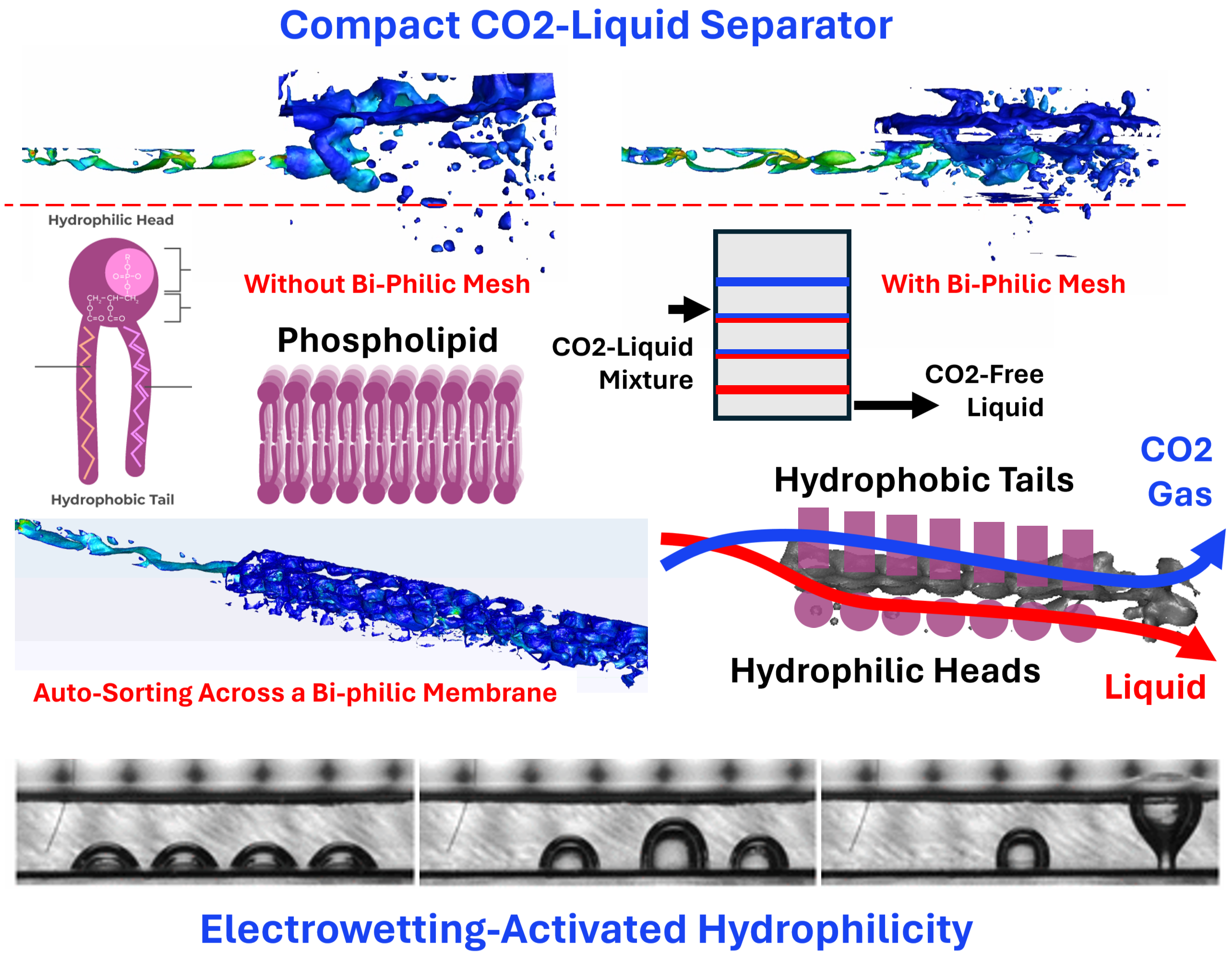
Traditional methodologies for separating CO2 from other gases and liquids are often ill-suited for the rapid, efficient, and compact operation required in urban-industrial contexts. Addressing this challenge requires a paradigm shift in our approach to separation technologies. Inspired by the principles underlying acceleration-based separation methods, such as gravitational or cyclonic separation, and the electrochemical processes involved in electrodialysis, our research proposes a novel application of bi-philic mesh technology. This technology draws a striking parallel between the ion-selective mechanisms of electrodialysis and the physical separation of gases from liquids, offering a path to enhanced efficiency and compactness in CO2 sequestration.
The Electrodialysis Parallelism
In electrolysis or electrodialysis, the separation of ions is created by an electric field, similar to the role of an acceleration field in gravity separation. The selective transport of charged species across ion-exchange membranes enhances the efficiency of electric-field, akin to the hydrophobic gas separation membranes. Just as electrodialysis employs bi-polar membranes—comprising a coupled anion-exchange and cation-exchange membranes—to efficiently separate and transport ions at reduced energy costs, our approach utilizes bi-philic meshes designed to selectively interact with gas and liquid phases. This selective interaction mirrors the natural separation observed in phospholipid bilayers, where hydrophobic and hydrophilic properties coexist to form highly efficient barriers and channels for transport within biological systems.
The innovation in our approach lies not only in the adaptation of electrodialysis principles to gas separation but also in the realization that these principles can be applied beyond the microscopic scale of ions and membranes. By developing surfaces that exhibit both hydrophilic and hydrophobic properties—achieved through advanced plasma-coating techniques and structural treatments—we extend the concept of bi-philicity to the macroscopic realm. These bi-philic meshes, unlike the thin and precise membranes used in electrodialysis, do not require the same level of finesse in their fabrication. Our research has demonstrated that even coarse meshes, with pore sizes tailored to the characteristic bubble sizes of the gas-liquid mixtures encountered in CO2 sequestration processes, can achieve remarkable efficiency in separating CO2 from other phases.
Operational Mechanics of Bi-philic Mesh Technology
Drawing a parallel to the functionality of bi-polar membranes in electrodialysis, our bi-philic mesh design ingeniously combines hydrophobic and hydrophilic properties to achieve selective separation of CO2 from exhaust streams. This dual characteristic is pivotal for the mesh's performance, mirroring the natural efficiency of phospholipid bilayers in biological systems. The hydrophobic sections of the mesh repel water, allowing gases to pass through, while the hydrophilic sections attract and pass water, effectively trapping CO2 for subsequent capture and storage. This segregation is facilitated by the inherent capillary action within the mesh structure, which exploits the instability of interfaces at the large surface tension differentials.
This principle of separation does not rely on the thin, precise construction typical of membrane technology but instead utilizes coarse meshes. Our Computational Fluid Dynamics (CFD) simulations reveal that even with 1-inch pore sizes, these meshes can dramatically enhance gas capture efficiency. In a comparative study focused on the CO2 separation process from thermal-transport fluids within a confined chamber, traditional gravity-based separation achieved a mere 94% efficiency, allowing a significant proportion of CO2 to escape back into the environment. However, when employing our bi-philic mesh setup, similar to an electrodialysis configuration, the efficiency leapt to 99%, showcasing the profound impact of this technology on improving CO2 capture in compact spaces.
Empirical Evidence and Comparative Advantages
The empirical evidence supporting the use of bi-philic meshes for CO2 capture is compelling. Beyond mere theoretical parallels with electrodialysis, the application of these meshes in a space-constrained environment demonstrated a leap in efficiency. This comparative advantage becomes even more pronounced when considering the operational scalability and energy efficiency of the system. The bi-philic mesh acts not just as a barrier but as an active participant in the separation process, utilizing the thermal-transport fluid's waste heat to enhance CO2 absorption and facilitate its transport to long-term storage solutions.
Furthermore, the architecture of the separation system incorporates a tripartite mesh setup: hydrophobic, bi-philic, and hydrophilic sections. This configuration emulates the stabilizing function of AEMs and CEMs in electrodialysis, ensuring that gas and liquid phases are effectively separated and stabilized before reaching the outlet. The bi-philic mesh, akin to bi-polar membranes, efficiently splits the two phases, exploiting high capillary gradients to disrupt any interface between them. This results in a characteristic phenomenon where gas is systematically stripped from the main flow as it passes through each section of the mesh, significantly reducing energy consumption and improving overall efficiency.
Advancements in Microscale Fluid Dynamics
At the microscale, our CFD models have provided further insights into the behavior of mixed liquid-gas fluids as they traverse bi-philic microchannels. These fluids naturally segregate into their respective phases, independent of gravitational forces, showcasing the intrinsic efficiency of the bi-philic design. This phenomenon is not merely a passive occurrence but a testament to the active sorting capability of the mesh, which leverages the unique interfacial properties of hydrophobic and hydrophilic sections to achieve separation. The dynamic interaction between gas bubbles and liquid droplets with these surfaces highlights a critical advantage of the bi-philic approach: the reduction of energy loss and inefficiency typically associated with viscous dissipation.
Moreover, the flexibility and adaptability of bi-philic surfaces offer unprecedented control over the separation process. Through electrowetting technology, it's possible to dynamically modulate the wettability of the mesh surface, thereby enhancing the separation efficiency and adaptability of the system. This capability to tune the interfacial characteristics of the mesh in real-time opens new avenues for optimizing CO2 capture processes, making it possible to adjust the system's performance based on varying operational conditions or gas compositions.
Conclusion and Future Directions
In conclusion, the development of bi-philic mesh technology represents a significant advancement in the field of CO2 capture and storage. By bridging the gap between traditional separation methodologies and the highly efficient principles of electrodialysis, we have unveiled a new paradigm in gas separation. This technology not only promises enhanced efficiency and compactness for urban-industrial applications but also offers a scalable, energy-efficient solution to one of the most pressing environmental challenges of our time. As we continue to refine and expand the applications of this technology, we anticipate broadening its impact across various sectors, further contributing to the global effort to mitigate climate change and promote sustainable urbanized industrial practices.
The Electrodialysis Parallelism
In electrolysis or electrodialysis, the separation of ions is created by an electric field, similar to the role of an acceleration field in gravity separation. The selective transport of charged species across ion-exchange membranes enhances the efficiency of electric-field, akin to the hydrophobic gas separation membranes. Just as electrodialysis employs bi-polar membranes—comprising a coupled anion-exchange and cation-exchange membranes—to efficiently separate and transport ions at reduced energy costs, our approach utilizes bi-philic meshes designed to selectively interact with gas and liquid phases. This selective interaction mirrors the natural separation observed in phospholipid bilayers, where hydrophobic and hydrophilic properties coexist to form highly efficient barriers and channels for transport within biological systems.
The innovation in our approach lies not only in the adaptation of electrodialysis principles to gas separation but also in the realization that these principles can be applied beyond the microscopic scale of ions and membranes. By developing surfaces that exhibit both hydrophilic and hydrophobic properties—achieved through advanced plasma-coating techniques and structural treatments—we extend the concept of bi-philicity to the macroscopic realm. These bi-philic meshes, unlike the thin and precise membranes used in electrodialysis, do not require the same level of finesse in their fabrication. Our research has demonstrated that even coarse meshes, with pore sizes tailored to the characteristic bubble sizes of the gas-liquid mixtures encountered in CO2 sequestration processes, can achieve remarkable efficiency in separating CO2 from other phases.
Operational Mechanics of Bi-philic Mesh Technology
Drawing a parallel to the functionality of bi-polar membranes in electrodialysis, our bi-philic mesh design ingeniously combines hydrophobic and hydrophilic properties to achieve selective separation of CO2 from exhaust streams. This dual characteristic is pivotal for the mesh's performance, mirroring the natural efficiency of phospholipid bilayers in biological systems. The hydrophobic sections of the mesh repel water, allowing gases to pass through, while the hydrophilic sections attract and pass water, effectively trapping CO2 for subsequent capture and storage. This segregation is facilitated by the inherent capillary action within the mesh structure, which exploits the instability of interfaces at the large surface tension differentials.
This principle of separation does not rely on the thin, precise construction typical of membrane technology but instead utilizes coarse meshes. Our Computational Fluid Dynamics (CFD) simulations reveal that even with 1-inch pore sizes, these meshes can dramatically enhance gas capture efficiency. In a comparative study focused on the CO2 separation process from thermal-transport fluids within a confined chamber, traditional gravity-based separation achieved a mere 94% efficiency, allowing a significant proportion of CO2 to escape back into the environment. However, when employing our bi-philic mesh setup, similar to an electrodialysis configuration, the efficiency leapt to 99%, showcasing the profound impact of this technology on improving CO2 capture in compact spaces.
Empirical Evidence and Comparative Advantages
The empirical evidence supporting the use of bi-philic meshes for CO2 capture is compelling. Beyond mere theoretical parallels with electrodialysis, the application of these meshes in a space-constrained environment demonstrated a leap in efficiency. This comparative advantage becomes even more pronounced when considering the operational scalability and energy efficiency of the system. The bi-philic mesh acts not just as a barrier but as an active participant in the separation process, utilizing the thermal-transport fluid's waste heat to enhance CO2 absorption and facilitate its transport to long-term storage solutions.
Furthermore, the architecture of the separation system incorporates a tripartite mesh setup: hydrophobic, bi-philic, and hydrophilic sections. This configuration emulates the stabilizing function of AEMs and CEMs in electrodialysis, ensuring that gas and liquid phases are effectively separated and stabilized before reaching the outlet. The bi-philic mesh, akin to bi-polar membranes, efficiently splits the two phases, exploiting high capillary gradients to disrupt any interface between them. This results in a characteristic phenomenon where gas is systematically stripped from the main flow as it passes through each section of the mesh, significantly reducing energy consumption and improving overall efficiency.
Advancements in Microscale Fluid Dynamics
At the microscale, our CFD models have provided further insights into the behavior of mixed liquid-gas fluids as they traverse bi-philic microchannels. These fluids naturally segregate into their respective phases, independent of gravitational forces, showcasing the intrinsic efficiency of the bi-philic design. This phenomenon is not merely a passive occurrence but a testament to the active sorting capability of the mesh, which leverages the unique interfacial properties of hydrophobic and hydrophilic sections to achieve separation. The dynamic interaction between gas bubbles and liquid droplets with these surfaces highlights a critical advantage of the bi-philic approach: the reduction of energy loss and inefficiency typically associated with viscous dissipation.
Moreover, the flexibility and adaptability of bi-philic surfaces offer unprecedented control over the separation process. Through electrowetting technology, it's possible to dynamically modulate the wettability of the mesh surface, thereby enhancing the separation efficiency and adaptability of the system. This capability to tune the interfacial characteristics of the mesh in real-time opens new avenues for optimizing CO2 capture processes, making it possible to adjust the system's performance based on varying operational conditions or gas compositions.
Conclusion and Future Directions
In conclusion, the development of bi-philic mesh technology represents a significant advancement in the field of CO2 capture and storage. By bridging the gap between traditional separation methodologies and the highly efficient principles of electrodialysis, we have unveiled a new paradigm in gas separation. This technology not only promises enhanced efficiency and compactness for urban-industrial applications but also offers a scalable, energy-efficient solution to one of the most pressing environmental challenges of our time. As we continue to refine and expand the applications of this technology, we anticipate broadening its impact across various sectors, further contributing to the global effort to mitigate climate change and promote sustainable urbanized industrial practices.
Acknowledgements