2024 AIChE Annual Meeting
(388e) Importance of Nanometer-Scale Bifunctional Interactions in Metathesis-Based Strategies for Polyolefin Deconstruction
Authors
Lucas Ellis - Presenter, University of Colorado - Boulder
Selena Moore, Oregon State University
Andrew Tran, Oregon State University
Andreas Palmateer, Oregon State Unversity
Jose Naranjo-Mendez, Oregon State University
Gatzios Dimitri, Oregon State University
Peter Eschbach, Oregon State University
Joel Miscall, NREL
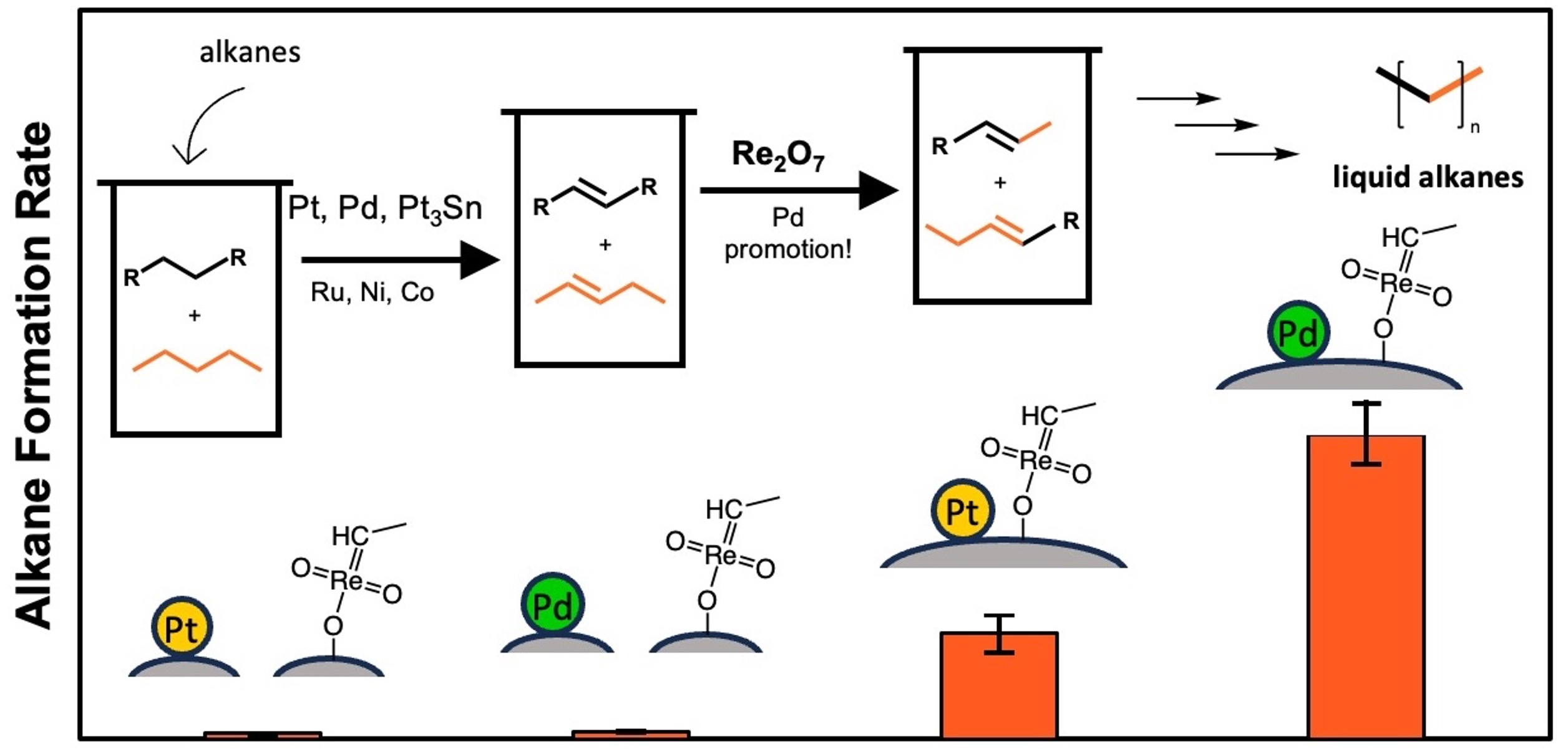
The rapid accumulation of plastic waste in landfills, waterways, and our oceans is resulting in a poorly understood environmental crisis, which current recycling technologies are not capable of ameliorating. Recent proposed approaches in the depolymerization of waste plastics employ an Olefin-Intermediate Process, where feedstocks like polyolefin plastics are ‘activated,’ producing an olefin intermediate. This olefin can then be reacted with an orthogonal and tandem chemistry, that results in new overall reaction pathways to alkanes or alkenes without the need for exogenous hydrogen. Here we investigated the role of the dehydrogenation catalyst on reaction rate, kinetics, and product distribution in heterogeneous tandem dehydrogenation and olefin metathesis, or alkane metathesis, of three different alkane reactants in both the liquid and gas-phase. We determined that a Pd-based dehydrogenation catalyst shows four-fold higher activity compared to a Pt or Sn1Pt3 catalyst on the same support. Uniquely, the method of catalyst preparation, demonstrated in-situ synthesis of a bifunctional catalyst capable of facilitating both dehydrogenation and olefin metathesis using alumina-supported metals and Re2O7, respectively. The Pd-based bifunctional catalyst demonstrated higher reactivity over time compared to the Pt-based catalyst and a surprising promotion of olefin metathesis activity. Kinetic investigations identified that the last reaction step, hydrogenation, appears to be the limiting this tandem chemistry. We show the Pd-based dehydrogenation catalyst displays marked improvement in the depolymerization rate of a linear polyethylene feedstock, compared to other demonstrated systems, with over 90% polymer conversion in 15 h at 190 °C using 33% less catalyst and increased reactant loadings. Lastly, we postulate that the use of naphtha range alkanes to depolymerize polyethylene feedstocks, in conjunction with a solvent recycle would result in reduced overall solvent consumption (i.e. reduced impact of solvent-solvent reactions).