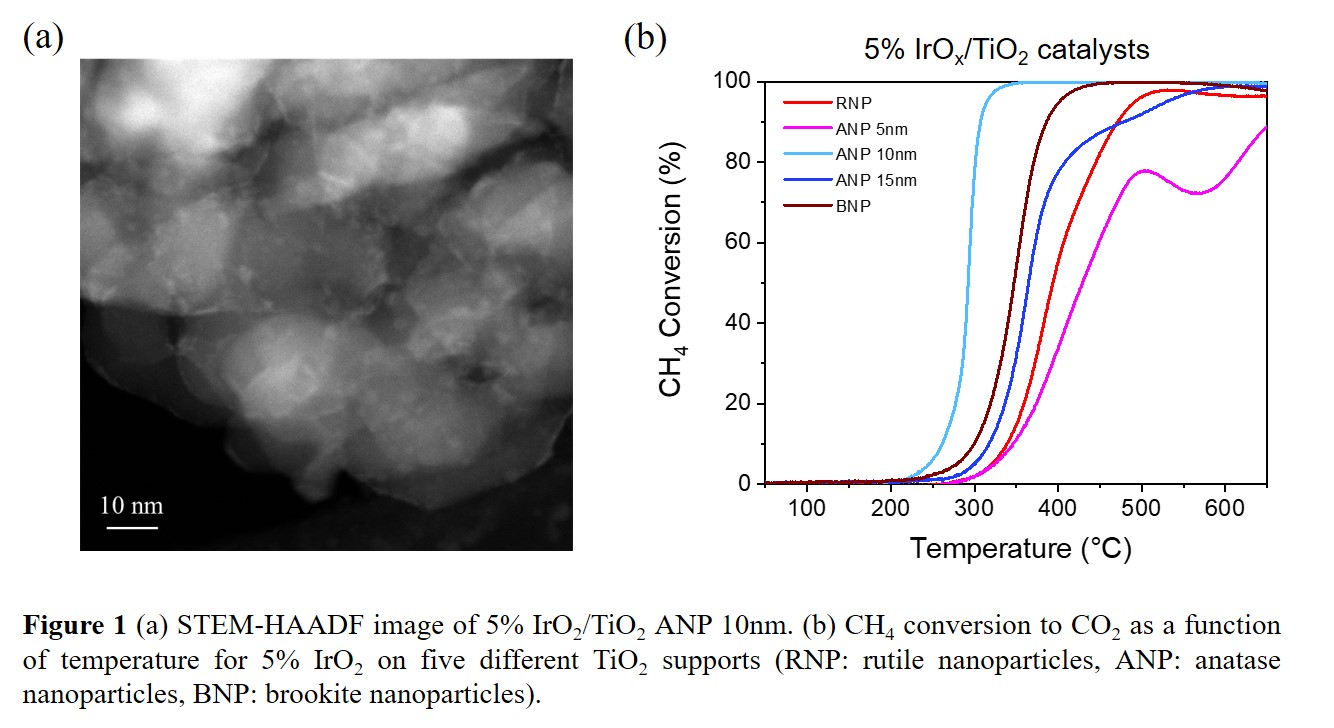
Reducing methane emissions is crucial for mitigating global warming, so developing highly efficient catalysts for low-temperature methane combustion is of great importance. Low temperature activation also has potential to facilitate selective oxidation of methane to higher value chemicals. Several noble or precious metals supported on various metal oxides, such as Pd/CeO
2 and Pd/TiO
2, are active in the methane combustion reaction and have been extensively studied. In contrast, despite the facile CH
4 activation observed over rutile IrO
2(110) surfaces, only a limited number of literature studies has focused on IrO
2-based methane activation catalysts. Our work has focused on TiO
2-supported IrO
x catalysts, and how the TiO
2 structure or crystal phase, namely rutile, anatase, and brookite, and titania particle size influence the activity in the methane oxidation. The different IrO
2/TiO
2 catalysts were synthesized using the urea deposition-precipitation method, followed by calcination in air at 350 °C. The catalytic performance of the IrO
2/TiO
2 in the methane combustion was then evaluated under excess oxygen conditions. Under these conditions, the activity is dependent not only on the titania structure, but also the titania particle size and of course the iridium loading. The best performing catalyst is IrO
x supported on the 10-nm anatase TiO
2 (
Figure 1), and we will also show that this catalyst is surprisingly stable with time on stream. This reveals that the structure and particle size of the support can significantly affect the catalytic performance in heterogeneous catalysts, and is very important in catalyst development by rational design.