Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Topical Conference: Waste Plastics
- Poster Session: Waste Plastics
- (385x) Acid-Catalyzed Decomposition of Polypropylene into Naphtha in Hydrocarbon Solvent

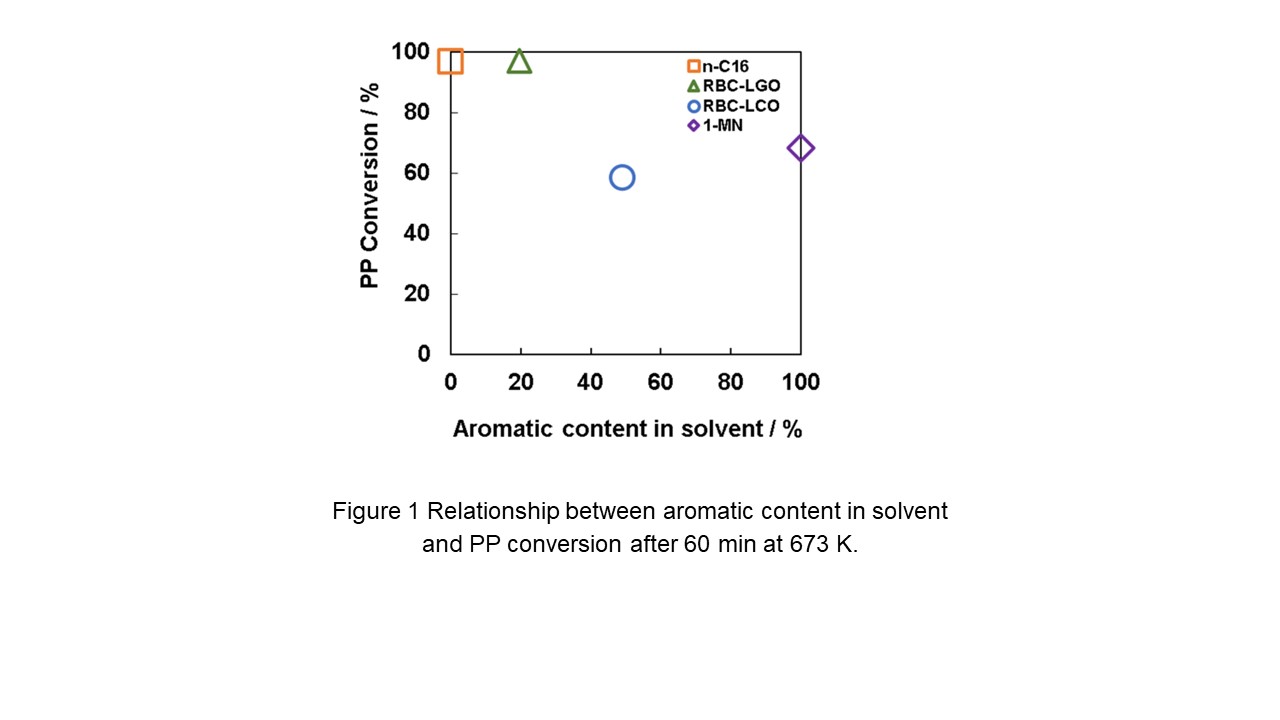
In this study, polypropylene (PP) was used as model plastic and zeolite beta as the catalyst. Hydrotreated light gas oil (RBC-LGO) and light cycle oil (RBC-LCO) were used as petroleum solvents. RBC-LGO had a higher content of aliphatic hydrocarbons, while RBC-LCO had a higher content of aromatic hydrocarbons. In addition, n-cetane (n-C16) and 1-methylnaphthalene (1-MN) were used as solvents representing linear alkanes and aromatic hydrocarbons. The decomposition behavior of PP in these petroleum solvents, e.g., PP conversion, was studied.
Decomposition test was conducted in a batch reactor at 673 K for 60 min using 20 g of solvent, 5 g of PP, and 1 g of beta zeolite. As shouwn Figure 1, while the levels of PP conversion was 97.0% and 58.4% when RBC-LGO and RBC-LCO were used as solvents, those were 96.9% and 68.2% when n-C16 and 1-MN were used as solvents, suggesting that aromatic hydrocarbons had a negative effect on the PP decomposition because the high aromatic hydrocarbon content in LCO inhibited the reaction of aliphatic hydrocarbons within the micropores of zeolite beta. The yields of C2-C9 petrochemical feedstock were 60-70%, depending on the reaction conditions, indicating that zeolite is a suitable catalyst for producing petrochemical feedstock. As a comparison, amorphous silica-alumina was also used as a catalyst, but the yield of petrochemical feedstock was low. Therefore, it was found that aliphatic hydrocarbon-rich solvents such as LGO are suitable for the cracking of PP.