Chabazite (CHA)-type zeolites offer significant potential in CO
2 separation from larger molecules, with applications in post-combustion carbon capture and natural gas/biogas upgrading. Their unique pore structure, approximately 0.37 × 0.42 nm^2 in dimensions, allows for precise discrimination between CO
2 (0.33 nm) and larger molecules like N
2 (0.364 nm) or CH
4 (0.38 nm), enabling selective separation.
The presence of siliceous constituents within CHA zeolites reduces adsorption capacity towards smaller molecules like H
2O (0.265 nm), resulting in decreased H
2O permeation rates. This hydrophobic nature ensures effective molecular sieving, maintaining excellent CO
2 perm-selectivity even in the presence of H
2O.
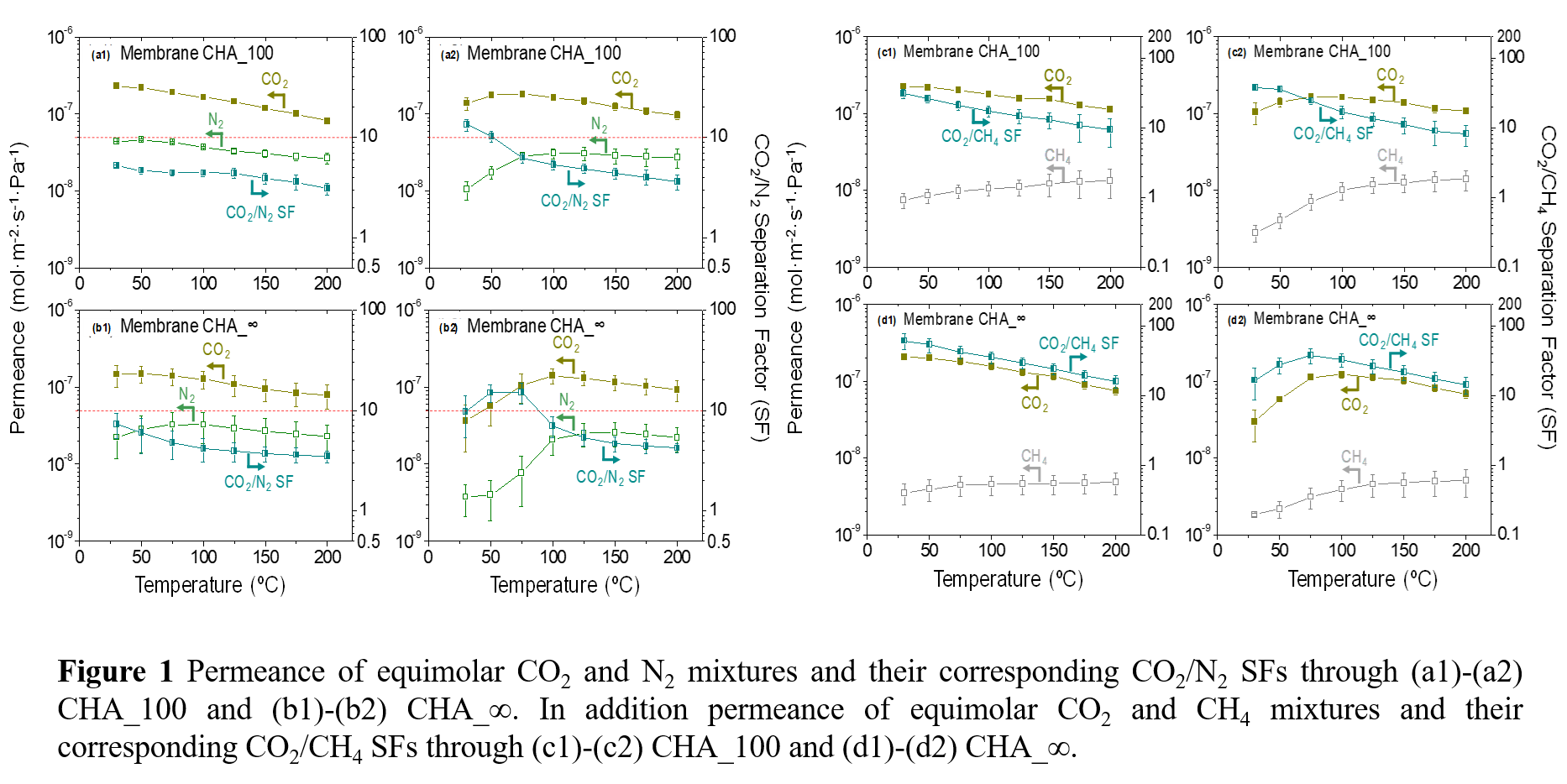
Experimental investigations on CHA_100 membranes with a Si/Al ratio of 100 demonstrated remarkable CO
2 separation performance, particularly in the presence of H
2O at 30 °C. The obtained results showed superior separation factors (SFs) for CO
2/N
2 (13.4) and CO
2/CH
4 (37) compared to dry conditions (5.2 CO
2/CH
4 SFs and 31 CO
2/CH
4 SFs, respectively). This improvement is attributed to physisorbed water molecules blocking defects.
A comprehensive quantitative analysis combining fluorescence confocal optical microscopy images and a one-dimensional permeation model revealed that approximately 19% and 20% of total CO
2 permeance for CHA_100 were hindered by physisorbed water molecules and defects, respectively.
In conclusion, CHA-type zeolites show promise for efficient CO
2 separation from larger molecules due to their molecular recognition capability and hydrophobic nature. Experimental results on CHA_100 membranes highlight superior CO
2 separation performance, with physisorbed water molecules playing a crucial role. Quantitative analysis provides insights into transport mechanisms, aiding the development of advanced CHA zeolite membranes for CO
2 separation applications.