2024 AIChE Annual Meeting
(363h) Hidden Chokepoints: Exploring Gas Diffusion Pathways in the Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS) Enzyme Complex Using Molecular Simulations.
Authors
CODH and CODH/ACS are central for atmospheric carbon monoxide oxidation and supporting microbial life. These enzymes are also the focus of recent biotechnological applications because of their potential replacement of two energy-intensive industrial processes: 1) the water–gas shift reaction, a widely used industrial process for H2 or CO production, and 2) the Monsanto process used to produce acetic acid from methanol and CO. However, the effective and widespread use of CODH and CODH/ACS for industrial fields is limited by their poor activity under aerobic conditions. One strategy to design O2-tolerant complexes is to prevent O2 from entering the enzyme matrix.
Our research framework is centered on addressing key questions, 1) the reaction mechanisms at the organometallic active sites, 2) the transport channels of the gas molecules within the proteins, and role of protein conformations in the overall process, and 3) the rate of CO2/CO interconversion in each direction of the channels that enter or leave the catalytic cycle.
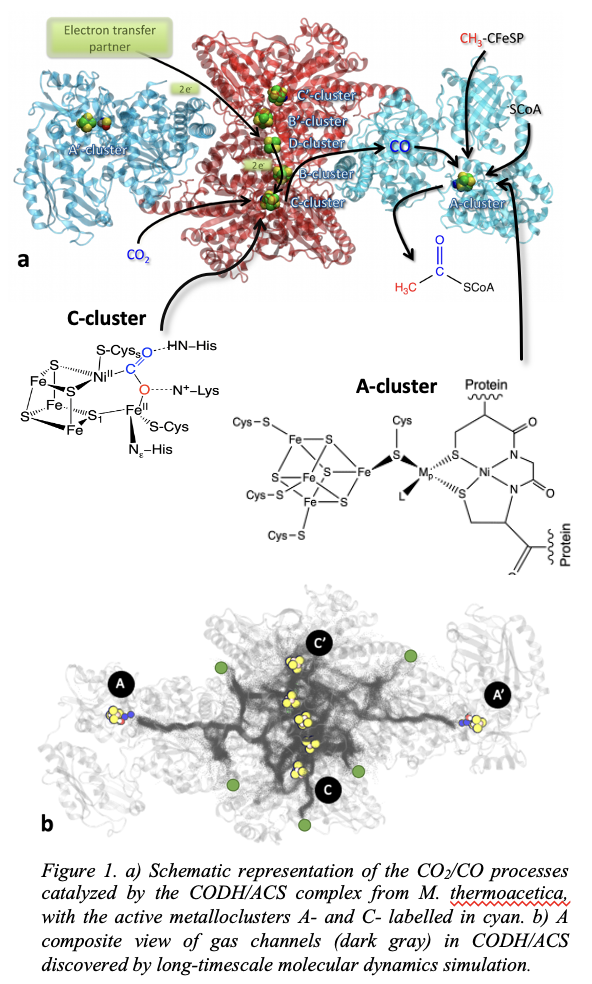
To address the fate of CO2 and CO upon reaching reaction sites, we employ density functional theory approaches to study the interaction of gas molecules at the active site within the protein environment. To elucidate the transport channels, we use atomistic free energy molecular dynamics simulations to explore the gas diffusion through the CODH/ACS complex from M. thermoacetica (Figure 1) and other design variants that favor uptake of CO2 and CO but restrict ingress of O2. We explore how the transport mechanism is affected by conformational changes in the subunits of the enzyme caused due to transformation of cavities and gating mechanism of residues. A hybrid Monte Carlo/Molecular Dynamics (MCMD) approach is used to investigate the electrocatalytic interconversions of CO2/CO at the active sites.
Research Interests:
“Peptoids” as a viable biomaterial in food packaging industry.
Antifreeze proteins (AFPs) stand as a paradigm-shifting innovation, that prevent freezing in organisms living in sub-zero environments by inhibiting ice crystallization. AFPs, such as Type I AFP from winter flounder and Type III AFP from ocean pout, have been instrumental in preventing ice crystallization in biological and industrial settings. This unique function has profound implications in cryopreservation, organ transplant technology, and notably in the food industry, where AFPs play a pivotal role in enhancing product longevity and quality.
Peptoids are synthetically engineered polymers, that could mimic natural AFPs with enhanced flexibility. The fundamental distinction between peptoids and peptides, while structurally similar, differ in the placement of their side chains. In peptides, side chains are attached to the alpha carbon atoms, whereas in peptoids, they are bonded to the nitrogen atoms of peptide backbone. Peptoids could be customized with targeted functional properties, to increase stability against enzymatic degradation, higher resilience under various physical conditions, and a reduced risk of immune responses, making them more suitable for industrial applications.
The research will address critical scientific questions: How do structural variations in peptoids influence their binding efficiency and antifreeze activity compared to natural AFPs? What thermodynamic and kinetic parameters are crucial in determining the antifreeze efficiency of peptoids? How can molecular dynamics simulations contribute to the design of optimized peptoid AFPs for industrial applications, particularly in food packaging?
Circular economy: Conversion of biomass-plastic waste to biochar-based adsorbents.
The crisis of plastic waste, particularly the accumulation of microplastics in the environment, presents a critical challenge for sustainable development. Addressing this issue requires innovative solutions that not only decompose plastic waste but also repurpose it into beneficial materials. Artificial metalloenzymes (ArMs) offer a targeted approach to catalyze the degradation of resistant plastic polymers, transforming them into value-added products such as biochar. Biochar, derived from the carbonization of organic matter, is recognized for its adsorptive properties, and is proposed mechanism to sequester pollutants.
Natural metalloenzymes like laccase and manganese peroxidase catalyze essential reactions in biological systems, including the degradation of complex molecules. Mimicking natural enzymes, artificial metalloenzymes can be engineered to target the sturdy chemical bonds in plastics such as polyethylene terephthalate (PET), high-density polyethylene (HDPE), and polystyrene (PS). Advanced molecular simulation techniques coupled with rational design and directed evolution can finely tune the active sites of metalloenzymes for specific substrate interaction, enhance catalytic rates, and provide stability against harsh industrial conditions.
The research aims to: Design artificial metalloenzymes with enhanced specificity for breaking down multiple types of plastics, focusing on the creation of intermediates destined for biochar conversion. Explore metabolic engineering of microorganisms to host these ArMs, evaluate the life-cycle of the enzymatic byproducts, and upscale the entire process for industrial use. Successful implementation would represent a dual achievement: mitigating plastic pollution and harnessing the resulting biochar for environmental adsorption applications, thereby fostering a circular economy in waste management.
PFAS-mediated dysregulation of biomolecular condensates.
The pervasive presence of per- and polyfluoroalkyl substances (PFAS) in the environment, coupled with their long biological half-life, has emerged as a concern for public health. PFAS compounds like PFOA and PFOS, notorious for their toxicity, exposure to it is linked to a multitude of health risks, and there is a critical need to understand their interaction with cellular components at a molecular level. This research focuses on the impact of PFAS on biomolecular condensates, which are crucial for various cellular processes, including RNA metabolism, signal transduction, and the stress response.
Biomolecular condensates, formed via liquid-liquid phase separation, are highly dynamic and sensitive to changes in their microenvironment. The physicochemical properties of PFAS, such as their hydrophobicity and stability, make them potential disruptors of the condensates' structural integrity. PFAS affect biomolecular condensates at the molecular level, this research aims to utilize molecular simulations to bridge the gap between environmental exposure and cellular dysfunction, offering a foundational perspective on the health implications of PFAS. This work has the potential to inform risk assessments, shape regulatory decisions, and inspire the development of therapeutic interventions to counteract the detrimental effects of PFAS on human health.