2024 AIChE Annual Meeting
(363ap) Catalyst for Direct Converting Carbon Dioxide to Alkanes/ Olefins
Author
Absract
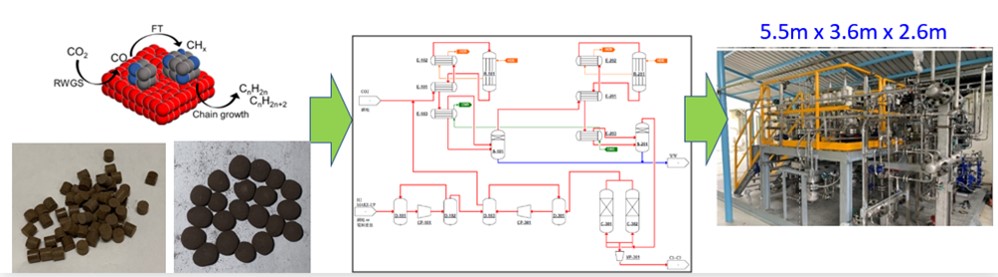
A novel catalyst was developed to directly convert carbon dioxide with hydrogen to liquid and gas alkanes/olefins. This catalyst is iron-based, and consists of porous FeO(OH)x ( Fe2O(OH)4 or FeO(OH) ) and alkaline metal compounds. The catalyst showed a high specific BET surface area over200m2/g, and exhibited a superior reactivity. Under the conditions of H2 to CO2 ratio of 3.5 and reaction temperature of 310°C, the carbon dioxide conversion reached 94.6% in the fixed bed reactor. The products were in both gas and liquid phases. In the gaseous products, the yield of carbon monoxide, methane, ethylene, ethane, propylene/propane, C4 olefins/alkanes, and C5-C7 olefins/alkanes was 1.3%, 20.8%, 8.9%, 4.7%, 17.6%, 10.7%, and 7.8%, respectively. On the other hand, there was C5+ olefins/alkanes with the yield of 22.8% in the liquid phase. During the 2000-hour test period, the catalyst activity remained stable, with no deactivation being observed. After 2000 hours of continuous reaction, the CO2 conversion rate was 94%. The key features of this catalyst includes a carbon dioxide conversion exceeding 90% and high selectivity towards olefins/alkanes, which increased the process economic benefits. This technology is currently at TRL7(System prototype demonstration in a space environment). The appearance of this prototype is shown in the figure below and the estimated production capacity is about one ton of olefins/alkanes annually. Since the hydrogen raw material cost accounts for more than 50% of the production cost of alkanes and olefins, hydrogen prices will be economically beneficial if they are below 2.0USD/kg. The total carbon emissions of every kilogram of alkanes and olefins produced is 1.55 kg CO2e/kg(based on 10kg CO2e/kgH2).