2024 AIChE Annual Meeting
(35b) Polymeric Nanotechnology Enables Versatile, Targeted Theranostics
Author
Jessica Larsen - Presenter, Clemson University
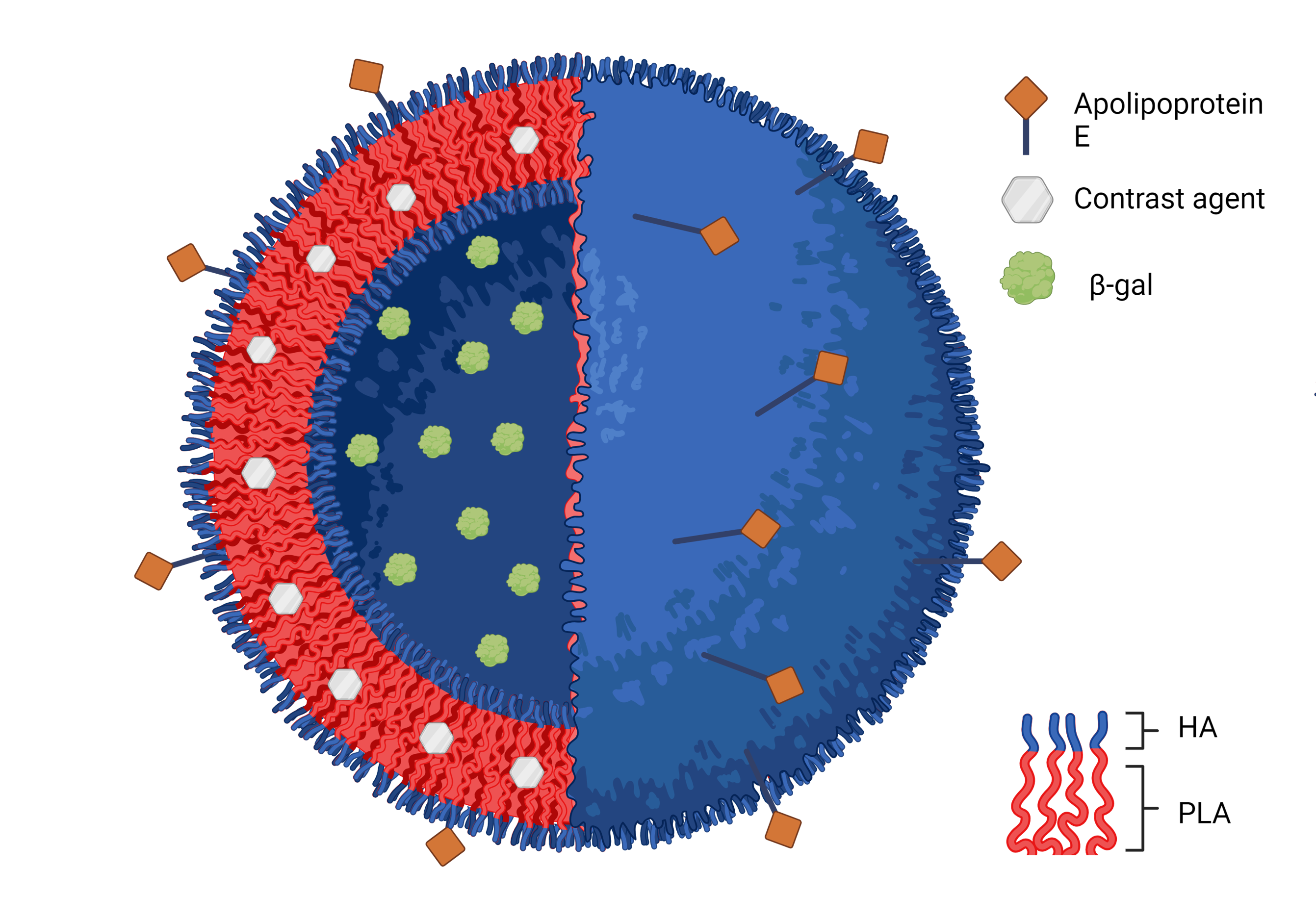
According to the World Health Organization, neurologic disorders account for the largest global burden through both early mortality and loss of independent life due to disability. Although there is a clear need for treatments that can enter the brain, there are a very limited number of medications available on the market. A major contributor to this fact is the presence of the blood-brain barrier (BBB), which prevents the passage of more than 98% of small molecule therapeutics from the blood into the brain tissue. The Larsen Lab works on polymeric biomaterials-based approaches to bypass and transport payloads through the BBB and the blood-nerve barrier. By understanding the pathophysiology of each disease, polymeric nanoparticles can be created to respond specifically to disease-based stimuli and promote natural healing processes in the brain. Polymersomes, self-assembled vesicles formed from amphiphilic block co-polymers, can enable simultaneous encapsulation and delivery of imaging agents and therapeutics, making them a valuable theranostic tool. In this talk, Professor Larsen will highlight some of her ongoing projects in this space, specifically focusing on the following: 1. Initial studies have developed enzyme-responsive polymersomes that can promote self-healing autophagic processes and enzyme restoration in neuropathic lysosomal storage disease model, GM1 gangliosidosis. Polymersomes made from hyaluronic acid (HA)-b-polylactic acid (PLA) are formed in a uniform size range using solvent injection formation techniques. Varying the HA molecular weight leads to control over release profiles of encapsulated enzyme beta-galactosidase. 2. These same enzyme-responsive polymersomes can co-deliver magnetic resonance imaging contrast agents with therapeutic proteins as an in situ neurologic tool. Furthermore, over time, degradation of HA-b-PLA polymersomes and release of the contrast agent leads to time-dependent loss of contrast. This demonstrates the potential for this tool to track drug release location. 3. Finally, pH-responsive polymersomes have been used as delivery vehicles for nerve-regenerative peptides isolated from the G3BP1 protein. These peptide-encapsulating polymersomes have been shown to promote neurite outgrowth in dorsal root ganglia cultures with neuronal specificity. Furthermore, the use of targeting ligands enables intramuscular injection in vivo in a rat model of sciatic nerve injuries. All of these projects demonstrate the versatility of the polymersome as a platform for important neural applications.