Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Nanoscale Science and Engineering Forum
- Bionanotechnology for Gene and Drug Delivery
- (338a) Engineered Encapsulins As Advanced Drug Delivery Platforms and Catalytic Nanoreactors

Protein nanocage-based biomedical delivery applications have so far mainly focused on safely and efficiently transporting protein- or RNA-based therapeutics into target cells. However, the possibility of combining two types of specific therapeutic macromolecules – protein and RNA – in a single nanocage design has not been explored, which can have several advantages over delivering them separately. To seize this opportunity, we have engineered encapsulin shells to capture functional RNAs while retaining their inherent protein loading capacity. Positively charged peptides were genetically fused to the N-terminus of the encapsulin protomer, displaying the peptides at the luminal surface of the encapsulin shell (Figure 2). This modification enabled encapsulins to efficiently and size-selectively package RNAs in vivo through electrostatic interaction during shell self-assembly. Furthermore, the engineered encapsulin was capable of simultaneous loading of multiple functional RNAs and most importantly, it was capable of concurrent packaging of RNAs and specific cargo proteins in vivo (Figure 3). After demonstrating the RNA and protein co-packaging capabilities, we proceeded to further improve them by enabling the targeted encapsulation of select RNAs in a sequence-specific manner in vivo. Since the aforementioned system solely relies on electrostatic interactions for RNA encapsulation, various endogenous RNAs were also captured inside the encapsulin shell along with RNAs of interest. In order to make the system specific regarding RNA encapsulation, we fused the λN-peptide known to strongly bind specific RNA sequences (19 nt RNA hairpin sequence: BoxB RNA) to the N-terminus of the encapsulin protomer, replacing the positively charged peptide of the precursor system (Figure 4). In such a modified system, target RNAs of interest can be specifically captured inside the encapsulin shell by tagging RNA with the BoxB RNA sequence at the genetic level. Strikingly, Next Generation Sequencing-based analyses revealed that the λN-peptide-fused encapsulin almost exclusively encapsulates the co-expressed target RNA while excluding endogenous RNAs (98% of the total encapsulated RNA was the target RNA). Compared to other protein nanocage systems engineered for targeted RNA loading, the λN-peptide-fused encapsulin system showed superior specificity in terms of target RNA encapsulation. What makes this system even more valuable is the fact that such specificity is attained in vivo, making disassembling and reassembling of protein nanocages in vitro for target RNA loading unnecessary, which is an essential step for many other RNA encapsulation systems. On top of λN-peptide-fusion, in order to ensure efficient therapeutic cargo delivery upon endocytosis, we are in the process of additionally introducing a pH-responsive amphipathic peptide (GALA) along with several histidine point mutations into the encapsulin shell, aiming to trigger shell disassembly at low endosomal pH. The resulting highly specific RNA and protein co-encapsulation system with the added benefit of low pH-triggered disassembly, has the potential for simultaneous co-delivery of bioactive RNAs and proteins, improving therapeutic outcomes.
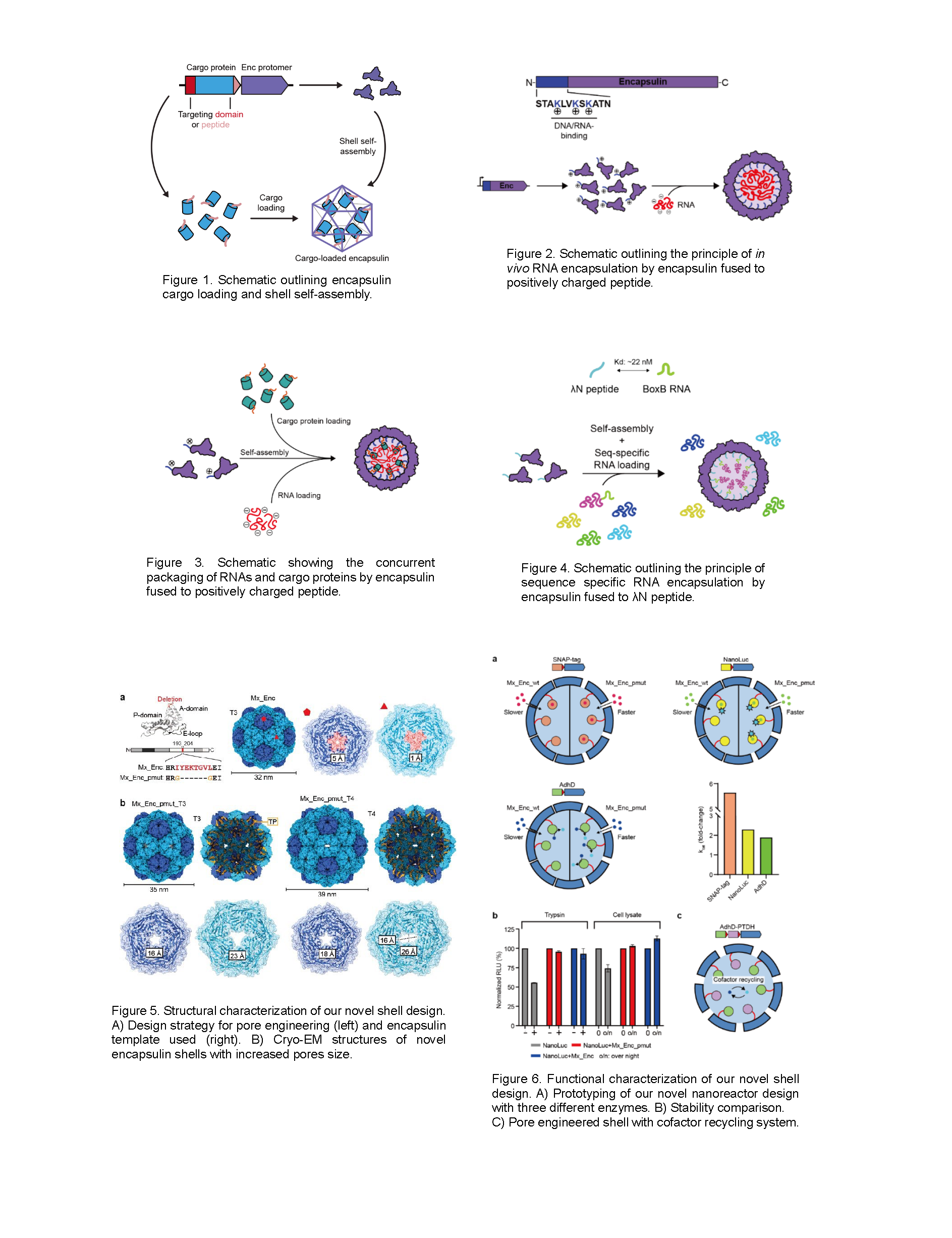
Encapsulins have also been exploited for the specific sequestration of various non-native enzymes with the goal of creating catalytically active nanoreactors with increased activity, stability, and longevity. However, the small native pores found in encapsulin shells often restrict the molecular flux of non-native enzyme substrates, cofactors, and products, decreasing the overall enzyme activity. Therefore, we engineered encapsulin shells with larger pores to allow more efficient access of non-native small molecules to the shell interior (Figure 5). In detail, we delete eight pore forming residues and added two glycine residues to allow flexibility. The resulting pore-mutated encapsulin (Mx_pmut) indeed improved the molecular flux across the shell, which was confirmed by SNAP-tag based fluorescence polarization (FP) assays. This improvement was characterized not only by faster molecular flux across the shell but also expansion of the upper size limit of substrate that can cross the shell, suggesting that a wider array of enzymes, particularly those utilizing large substrates or cofactors, can now be utilized with Mx_pmut based nanoreactors. This capability represents a significant advancement, as such enzymes could previously not be used with wild-type encapsulins due to substrate/cofactor inaccessibility. To demonstrate these improvements, we designed and tested two prototype nanoreactor systems based on a luciferase (NanoLuc) and a promiscuous alcohol dehydrogenase (AdhD), utilizing the relatively large substrate fluorofurimazine and redox co-substrate NADH, respectively (Figure 6). We showed that the turnover number for both enzymes significantly increased in Mx_pmut-based nanoreactors compared to wild type encapsulin (Mx_wt)-based nanoreactors. Lastly, we demonstrated the ability to co-encapsulate and produce functional multi-enzyme systems in Mx_pmut based nanoreactors. Specifically, we successfully implemented an efficient cofactor recycling system within Mx_pmut by co-encapsulating AdhD and the phosphite dehydrogenase PTDH (Figure 6). The improved performance consistently shown in nanoreactor systems based on Mx_pmut underscores the significance of pore size in determining overall nanoreactor performance, likely through controlling the molecular flux across the shell and thus the accessibility of substrates/cofactors and the release of products.
One of the potential concerns with increasing pore size is the loss of structural integrity and protective capacity of the shell. It has been shown that pores larger than >3 nm can allow small proteins like RNase to access the luminal space of the cage resulting in the loss of the protective capacity of the shell. Dynamic light scattering analyses, coupled with thermal ramp stability tests, showed that deleting pore-forming residues in Mx_pmut does not compromise the stability of the shell. Furthermore, encapsulated enzymes in Mx_pmut retained their activity upon proteolytic and bacterial cell lysate challenges, highlighting that the optimized porosity in Mx_pmut shells does not diminish their protective function (Figure 6).
In summary, we have engineered encapsulin nanocages to yield novel and versatile encapsulin-based drug delivery and high performance enzyme nanoreactor platforms with a broad future application range spanning biomedicine, biocatalysis, and bionanotechnology.