Musculoskeletal conditions and injuries present a common, costly, and chronic challenge to the over 1 billion people affected worldwide. Such issues can severely limit quality of life, making musculoskeletal conditions a leading case of disability in the world. With over 4.5 million annual physician visits, shoulder pain represents the third most common type of musculoskeletal injury, and such pain is often associated with injury to the rotator cuff, a condition that remains a clinical challenge to repair. The rotator cuff connects soft tissue (muscle, tendon) to hard tissue (bone) through a fibrocartilaginous transitionary region known as the enthesis, providing critical mechanical support by dispersing stress and thus reducing potentially damaging stress concentrations. Unfortunately, following a tear, the highly structured and supportive fibrocartilaginous enthesis can be replaced with disorganized fibrovascular scar tissue. This tissue is more susceptible to inflammation and far weaker than the original tissue, heightening the risk of re-tear. While surgery can reattach torn tendon back to bone, it is not well-equipped to facilitate regeneration of the native enthesis, resulting in frequent surgical re-failure, reported as high as 94% in large and massive tears. Such challenges necessitate the need for next-generation techniques of rotator cuff repair, and we are developing a biomaterials-based approach to promote regeneration of the native enthesis. Our design integrates instructive tendon- and bone-mimetic scaffolds via a thiolated gelatin (Gel-SH) hydrogel, which mechanically supports the transition between the two. This space also offers a potential region of biological transition, and we have hypothesized that human mesenchymal stem cells (hMSCs) cultured within the osseous and tendinous regions of the material could influence cellular behavior within the region toward an entheseal phenotype, as the importance of cellular crosstalk in interfacial regions has been reported in both enthesis development studies and
in vitro analysis of interfacial cell triculture. However, the current iteration of this design does not match the toughness of the physiological enthesis or make for easy handling in surgical procedures; additionally, biological responses of hMSCs within would benefit from more precise, potent, and timely signaling rather than relying purely on hMSC activity. Here we outline our efforts to improve both the mechanical support offered by this hydrogel within the overall material and the biological cues it provides in guiding a fibrocartilaginous enthesis phenotype.
Multicompartment scaffolds are fabricated through the lyophilization of precursor solutions layered within a custom Teflon-copper mold. Precursors for the tendinous and osseous zones are created by homogenizing a slurry of collagen and chondroitin sulfate, as well as other salts and minerals, in an acidic buffer solution. Gelatin is thiolated by Traut’s reagent, dialyzed, lyophilized, and used to create a 3.5 wt% hydrogel precursor. Crosslinking is achieved enzymatically, using tyramine and horseradish peroxidase to oxidize thiol groups on the gelatin backbone, leading to the formation of disulfide bonds. The collagen slurries and hydrogel precursor are loaded into the mold, allowed time for hydrogel diffusion and crosslinking, and lyophilized; a copper wall facilitates unidirectional heat transfer on one side, creating aligned, elongated pores in the tendinous area of the scaffold while the osseous region retains an isotropic pore structure. Multicompartment material properties are evaluated via SEM imaging, pore size analysis, and uniaxial tensile testing. For in vitro work, scaffolds are sterilized via ethylene oxide treatment, hydrated in phosphate buffered saline, and exposed to additional crosslinking (EDC/NHS carbodiimide chemistry) before incubating in cell culture media. Human mesenchymal stem cells (P4-P5, RoosterBio) are cultured in flasks, seeded onto hydrated scaffolds, and evaluated over periods between 7 and 28 days for cell number (DNA isolation), metabolic activity (alamarBlue assay), gene expression (PCR or NanoString nCounter), and protein expression (Western Blot, ELISA, etc.). Such methods allow us to consider modifications to this system that improve overall scaffold stability and mechanics as well as bioactivity and relevance in enthesis tissue engineering.
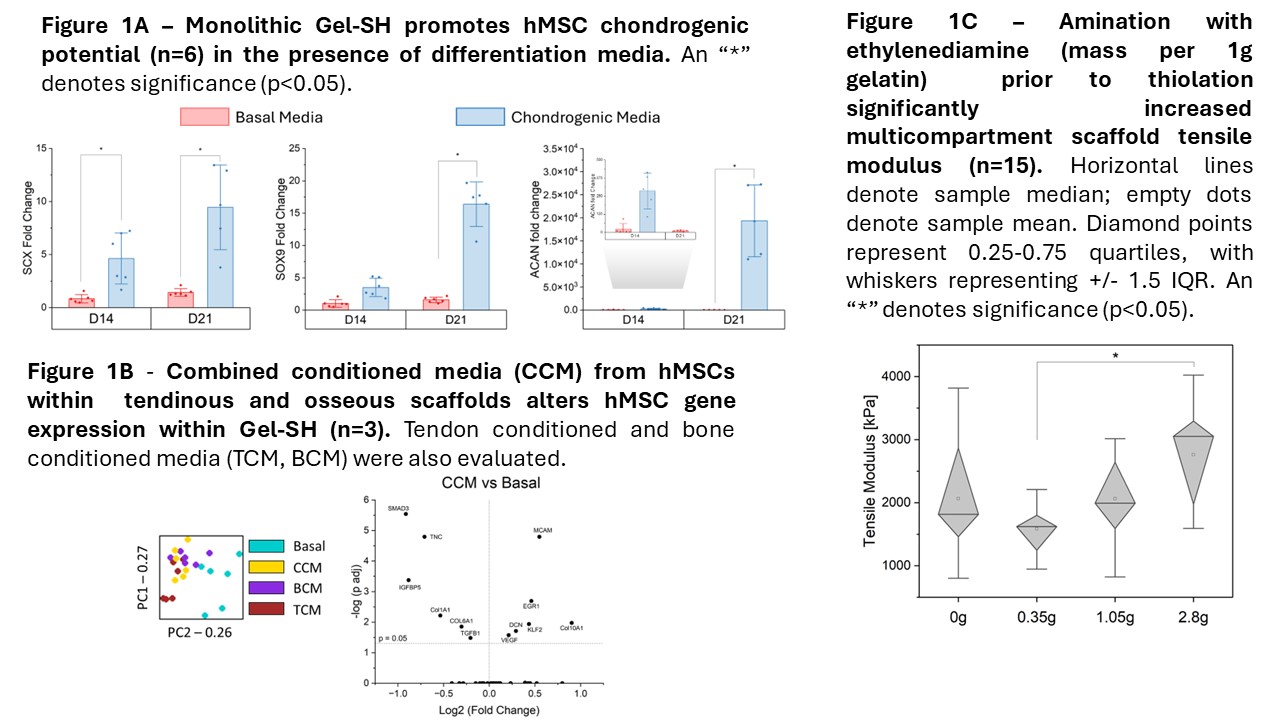
Biologically, we have demonstrated that this gelatin platform supports chondrogenic potential with the proper signaling (Fig. 1A), that hMSCs in neighboring osseous and tendinous compartments generate signals that significantly influence hMSC behavior within Gel-SH (Fig. 1B), and that this influence is also observed in the full multicompartment material. We are currently considering the incorporation of other naturally derived extracellular matrix components (chondroitin sulfate, collagen 2) to further enhance fibrochondrogenic capability of the material beyond the established baseline, and we are investigating the use of hMSC extracellular vesicles to strengthen tendinous and osseous tissue signaling. These modifications could allow for quicker and earlier signaling to occur, independent from the signals generated from seeded hMSCs. From a mechanical standpoint, we are exploring the use of ethylenediamine to induce greater amination of native gelatin, providing more reacting groups for the Traut’s reagent to thiolate and potentially resulting in greater material strength and toughness. Preliminary data (Fig. 1C) suggests that fabrication with this pre-aminated Gel-SH results in a stiffer overall multicompartment material. Through these modifications, we aim to develop a tougher material that provides faster and clearer signaling for hMSC differentiation, allowing for functional regeneration of the native enthesis for improved outcomes in rotator cuff repair.