Hitherto catalytic approaches to maintain resource circularity of plastics utilize the learnings in hydrocarbon chemistries and often use noble metals for the C-C bond cleavage for converting polymers into chemicals, and fuels. Reductive methods requiring high-pressure H
2 (>20 bar) have emerged as promising low-temperature routes for their chemical deconstruction. However, H
2 is expensive and derived from fossil sources. In 2021, only 1% of H
2 was classified as low emissions. As such, H
2-free routes are desired to deconstruct plastic wastes effectively and sustainably. H
2-free approaches for condensation polymers, of which the polyester, polyethylene terephthalate (PET) alone comprises of 10% of the total waste, includes chemolysis (e.g.,alcoholysis) of ester bonds with the use of excess solvents (e.g.,methanol) and homogenous catalysts.
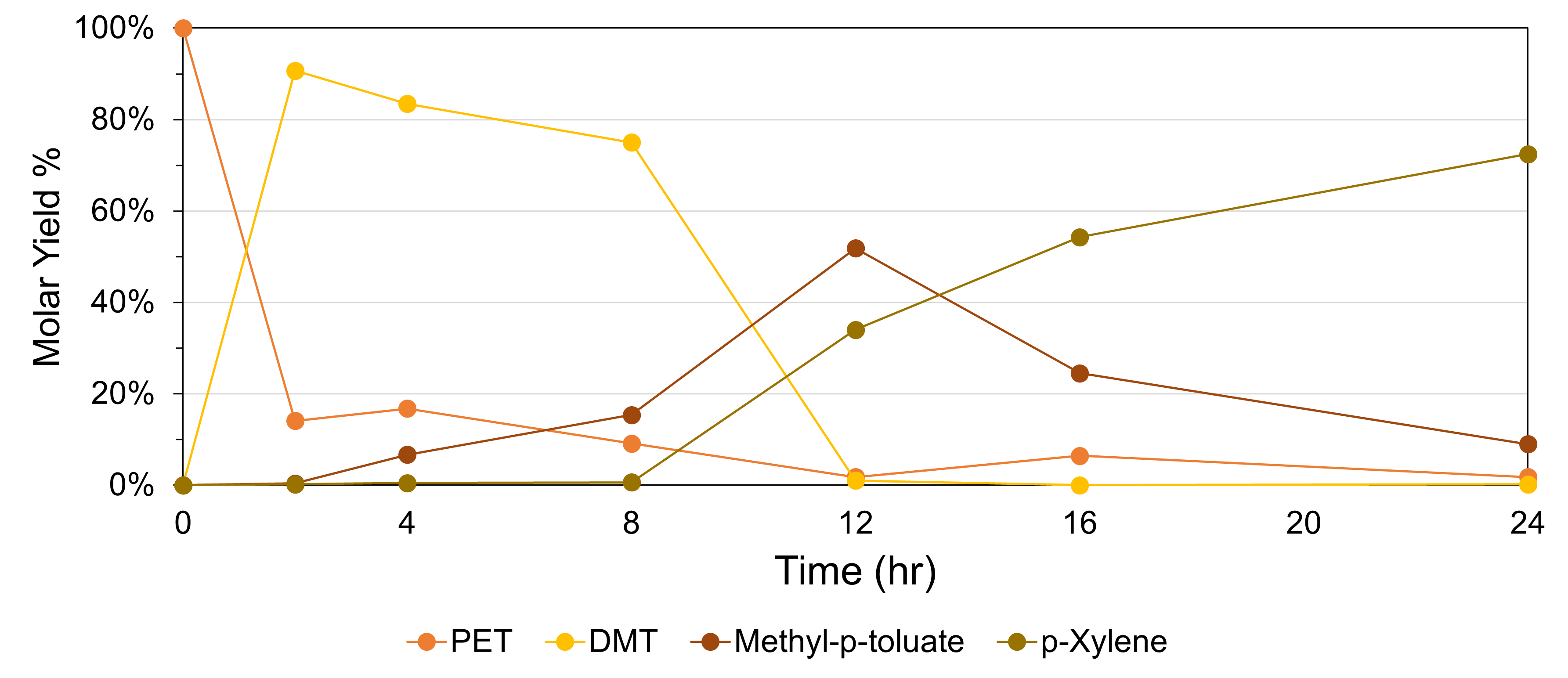
In this work, we utilized CH3OH as a solvent and H-donor on metal-oxide interfaces of Cu metal on reducible metal-oxide (e.g.,Zn0.2Zr0.8O2) to kinetically couple dehydrogenation of CH3OH and hydrogenolysis of dimethyl terephthalate (DMT) to p-xylene. We investigate the role of direct and indirect H-transfer with reactivity analysis and density functional theory (DFT) computations. This was coupled with microscopic and spectroscopic investigation with TEM and SEM, XPS, EPR, and H2-TPR to explicate the role played by the interface on the mechanism and kinetic coupling.
Table 1 shows that the interface of Cu on a Zn0.2Zr0.8O2-ö support (not Cu or the Zn0.2Zr0.8O2-ö support alone) shows activity for the hydrogenolysis of DMT to p-xylene. Our mechanistic hypothesis is further supported by the fact that under our reaction conditions where CH3OH is in the gas-phase, the duality of CH3OH is broken, and it functions solely as a H2 carrier (Figure 1).
Table 1. Catalytic activity for CH3OH dehydrogenation, DMT conversion, and H2 formation in the gas-phase.
| Catalyst |
CH3OH consumption |
DMT conversion |
Final H2 formation |
| Cu/SiO2 |
18% |
0% |
3% |
| Zn0.2Zr0.8O2 |
2% |
0% |
0% |
| Cu/Zn0.2Zr0.8O2 |
46% |
100% |
21% |