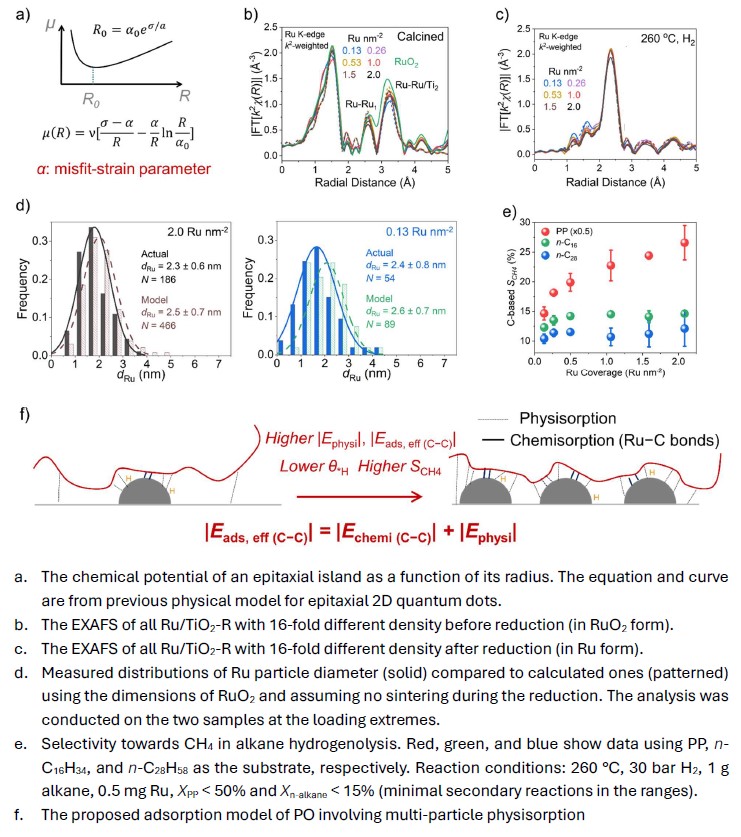
In heterogeneous catalysis, the density of active metal particles is a crucial parameter that affects the transport of spilled species, the sintering and electronic state of active sites, and the coverage, thus confinement of intermediates. Nevertheless, particle density effects are difficult to elucidate because they are usually convoluted with particle size effects. Here we will present a new approach leveraging epitaxial growth to break the correlation between particle size and density. Combining EXAFS and STEM analysis, we will show that at sub-monolayer coverages, RuO
2 grows on rutile TiO
2 epitaxially as strain-limited islands with coverage-independent size (Fig. a: simulated critical radius; Fig. b: the EXAFS), a growth mode previously observed in self-assembled quantum dots. The size of the islands is inherited by metallic Ru particles formed after the reduction (Fig. c: the EXAFS; Fig. d: STEM distribution, measured vs. predicted from dimensions of RuO
2), leading to their constant size and shape (
d ~2.3 nm, three-dimensional) with 16-fold different density. The epitaxial growth approach was deployed to investigate polyolefin (PO) hydrogenolysis, in which high Ru particle density increases the selectivity towards CH
4 (Fig. e) Such effect is absent when small alkanes that can be accommodated on one Ru particle are used as the substrate (Fig. e), and attributed to the strong multi-particle physisorption of PO (Fig. f) shifting the competitive adsorption with *H, thus lowering *H coverage. Mechanistic difference between PO and small-alkane substrates will also be presented. This work provides a novel general strategy to deconvolute particle density and size effects that is applicable to thermal catalytic reactions under harsh conditions.