2024 AIChE Annual Meeting
(293b) E-Heated Ethanol Dehydration for Sustainable Ethylene Production
Authors
1. Introduction
The substantial reliance on fossil fuels to meet global energy demands poses a significant obstacle to achieving climate neutrality targets [1]. A profound shift is necessary, encompassing the adoption of renewable energy sources, advancements in heating technologies, and the utilization of regenerative feedstocks. Electrified catalytic reactors, leveraging technologies such as resistive, inductive, and microwave heating, offer a promising way to expedite this transition. While extensive research has delved into the resistive heating of electrically conductive wall reactors and structured catalysts in high-temperature applications, particularly in Steam-Methane-Reforming [2], reverse-Water-Gas Shift [3] and ammonia decomposition reactions [4], limited attention has been given to resistively heated catalytic fixed-bed reactors [5,6]. Implementing resistive heating in a catalyst bed consisting of granules/particles is technically challenging due to the need for physical continuity and control over the contact area between conductive particles to prevent hot spot formation [7]. Nonetheless, on industrial scale, catalytic fixed-bed reactors involving granules/particles remain prevalent [8]. Our goal is to electrify endothermal (bio)ethanol-to-ethylene reaction, optimizing control through an electrically heated fixed-bed approach for improved economic and ecological impact. The integration of regenerative feedstocks with energy-efficient heating technology aims to achieve sustainable olefin production.
2. Methods
A tube reactor was utilized to evaluate the bed resistivity of a physical mixture across the axial length of the bed. Variations in volume fractions and particle size were conducted to identify the optimal parameter for achieving a sufficiently conductive and reproducible bed. The catalyst bed comprised a homogeneous physical mixture of electrically conductive graphite particles and commercial alumina catalyst. Catalytic investigations, incorporating resistive heating, were conducted in a nearly adiabatic double-tube reactor with radial heat insulation, suitable for a 5 ml fixed-bed configuration. Further investigations involved scaling up the fixed-bed reactor from 5 ml to 500 ml capacity.
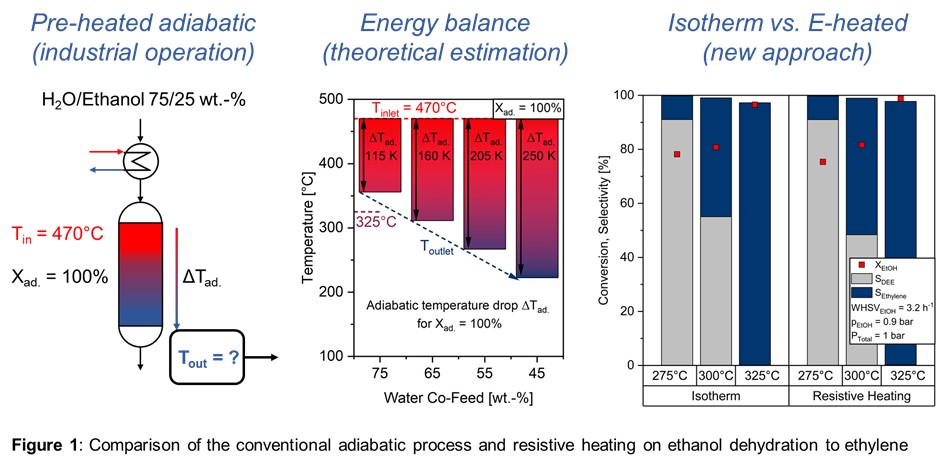
3. Results and discussion
Resistivity measurements showed that achieving the targeted bed resistivity requires a volume fraction of approx. 30% of conductive particles. The successful demonstration of resistive heating in an electrically conductive catalyst bed for endothermal ethanol dehydration showed that nearly full conversion of ethanol to ethylene could be achieved at 325°C. Comparing to the reference isothermal kinetics, the homogeneous temperature distribution is evident, indicating the lack of detectable hotspots. In contrast to the industrial adiabatic process [9], which uses 75 wt.-% water in the feed as a heat carrier to prevent significant axial temperature gradients, the feed in e-heated approach consists of pure ethanol, as illustrated in Figure 1. Consequently, significant heat losses in the heat carrier gas are inevitable. Additionally, resistive heating of the catalyst bed allows operation at a significantly higher weight hourly space velocity (WHSVEthanol) at a comparable gas retention time compared to the industrial process with a large amount of water, responsible for high gas velocity. The successful demonstration of scaling up from 5 to 500 ml was achieved, with axial temperature profiles being recorded for different feed compositions, covering both high and low water content. The nearly absence of water have shown lower temperatures in the catalyst bed, since kinetical inhibiting of water [10] and higher gas velocities can be eliminated with resistive heating of catalytic fixed-bed. Since water as heat carrier is not needed anymore, less energy input was observed.
4. Conclusions
In the conventional adiabatic process, the performance is influenced by the heat supplied through gas pre-heating, whereas in case of resistive heating, performance is kinetically controlled. Therefore, the reaction is driven to the catalyst’s capacity limit, enhancing catalyst utilization. While the pre-heated process is not able to achieve the target performance in the absence of water, e-heated approach demonstrates the ability to operate in omission of heat carrier. Eventually, resistive heating can enable to run endothermal reactions emission-free, simultaneously increase productivity and process energy-efficiency by preventing heat losses in heat carrier. All in all, resistive heating can lead to intensify the process and decarbonize the heating of endothermal reactions.
References
[1] IEA (2021), Key World Energy Statistics 2021, IEA, Paris https://www.iea.org/reports/key-world-energy-statistics-2021, License: CC BY 4.0.
[2] S.T. Wismann, J.S. Engbæk, S.B. Vendelbo, F.B. Bendixen, W.L. Eriksen, K. Aasberg-Petersen, C. Frandsen, I. Chorkendorff, P.M. Mortensen, Science (New York, N.Y.) 364 (2019) 756–759.
[3] L. Zheng, M. Ambrosetti, A. Beretta, G. Groppi, E. Tronconi, Chemical Engineering Journal 466 (2023) 143154.
[4] A. Badakhsh, Y. Kwak, Y.-J. Lee, H. Jeong, Y. Kim, H. Sohn, S.W. Nam, C.W. Yoon, C.W. Park, Y.S. Jo, Chemical Engineering Journal 426 (2021) 130802.
[5] Soderquist et al., An improved process for the catalytic dehydrogenation of hydrocarbons, 1968.
[6] Y.R. Lu, P. Nikrityuk, Applied Energy 228 (2018) 593–607.
[7] L. Zheng, M. Ambrosetti, E. Tronconi, ACS Eng. Au (2023).
[8] R.J. Wijngaarden, A. Kronberg, K.R. Westerterp, Industrial catalysis: Optimizing catalysts and processes, Wiley-VCH, Weinheim, 1998.
[9] L. Roza, E. L. Faleiros, Method for producing olefines from ethanol fuel, 2014.
[10] J.F. DeWilde, H. Chiang, D.A. Hickman, C.R. Ho, A. Bhan, ACS Catal. 3 (2013) 798–807.