2024 AIChE Annual Meeting
(281d) Free-d Molding – Every Idea Deserves a Prototype
Major factors are (1) resources, (2) formulation development, and (3) process development.
The resource challenge gets tougher when working on new formulations. New Chemical Entities (NCE) can be expensive and unavailable to start a regular 3D printing project. The reason is simple. Conventional 3D printing usually requires closely defined material parameters, e.g., 1.75 mm filament diameter (+/- a few 1/100 mm) or a powder with defined particle size distribution and flowability. To obtain applicable starting materials, intensive upstream process development is a necessity. Thus, projects often end before they have a real chance to prove their success.
The formulation challenge is to choose the proper excipients from the many available options. Many vendors offer a wide variety of different polymers with different molar mass distributions or chemical modifications, each of which shows other material characteristics when it comes to processing, drug stability, and release; thus, many trials are necessary to identify the best carrier material. Running those trials with material-hungry extrusion equipment will waste a lot of material and valuable time as new formulations require time to find suitable processing parameters.
This is where the process development challenge starts. Small changes in API concentration can influence the material properties, causing the whole process to change its behavior. Finding the correct process parameters to achieve high product quality requires a lot of experience and material understanding, which is not available during the birth of an idea.
Efficient development is only possible when the complex development challenge is broken into two pieces: formulation development and process development. This reduces the complexity as interactions between both sets of challenges are minimized. The formulation challenge is the first to solve. Only if the formulation works, meeting target dissolution profiles, stability data, and other relevant product attributes, the processing challenges are worth solving. They are less complex as the formulation composition is well-defined and remains constant, and the process can be optimized for this specific case.
For the formulation development of simple geometries (e.g., discs, cylinders) Vacuum Compression Molding (VCM) has been established over the last few years to screen for amorphous solid dispersions [2] and drug-eluting implants. It is a powerful formulation screening tool for creating prototypes with little material knowledge. It mimics hot melt extrusion by the lossless melting of a homogeneous powder in an evacuated, adaptable sample chamber with minimal process parameters.
This case study demonstrates how the benefits of conventional 3D printing can be facilitated to generate molds for the VCM technology. The molds are inserted in the VCM chamber, and arbitrary shapes can be obtained with minimum process development as only processing temperature and time are necessary. This new method is called free-d molding, as researchers’ ideas are freed [3].
METHODS
The first step starts on the computer by designing a 3D model of the desired dosage form. In the example below, a gummy bear is chosen. Then, straightforward 3D printing with engineering-grade resins creates positive models of the desired geometries. A non-stick material is used to cast negative molds from the 3D-printed parts, which can be placed inside the VCM Tool (MeltPrep, Austria). Since the whole VCM process operates under a vacuum, there is no air to displace inside the cavities of the molds. When heated, powdery materials fuse into melts, which fill the cavities. A detailed formulation prototype is created while running the conventional VCM process. Figure detail A shows a typical timeline for a free-d molding project from the idea to a new formulation prototype.
Depending on the shape and functionality of the final dosage form, three different types of free-d molding can be applied.
- For imprint free-d molding, a non-stick mold is inserted into the VCM Tool and filled with the powdery formulation. During the VCM process, the surface imprint on one side of the sample adapts to the used template, while the backside stays flat, forming a homogeneous disc. The amount of filled material adjusts the height of the backing.
- Split-cavity free-d molding uses two non-stick mold halves, forming one cavity with a gate facing towards the feed chamber holding the powdery formulation. During the VCM process, the formulation melts, flows through the gate, and fills the cavity to form any 3D geometry. The remaining material in the gate is called sprue and is removed with a clipper.
- Multi-component free-d molding is a multi-step process and can combine split-cavity and imprint free-d molded geometries. Various specimens from previous prototyping steps are fused to create one final formulation prototype.
RESULTS
To show the applicability of free-d molding, various matrix formulations and multi-component dosage forms used in the pharmaceutical field are tested. Feature sizes range from micrometers, enabling geometries such as microneedles with sharp tips, up to centimeters, allowing prototyping of floating devices or rings.
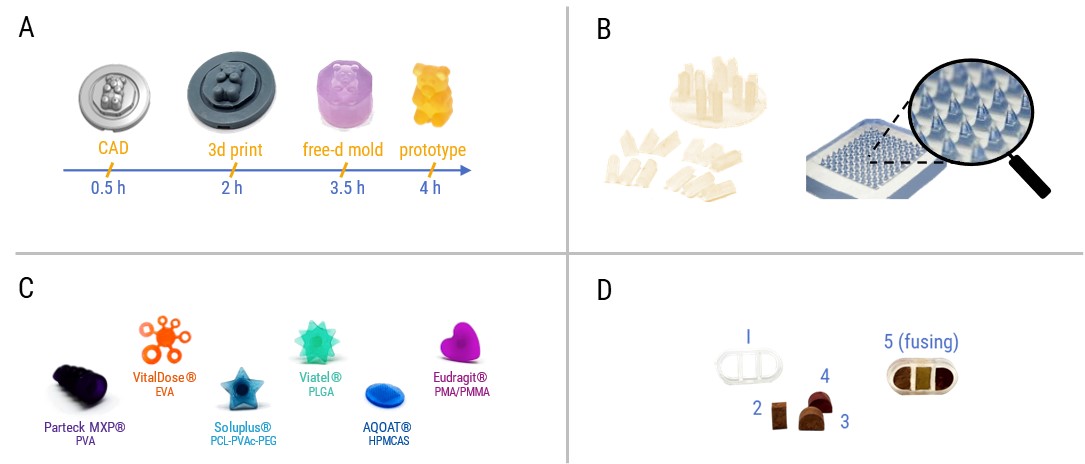
- Imprint Free-d Molding is used to demonstrate the prototyping of subcutaneous implants (Figure detail B, left). A batch with multiple rods was created in less than 15 minutes. Cutting off the implants with a flush cutter gave access to the individual drug-eluting implants with various cross-sections. Microneedle templates (Smicna, Singapore) enabled melt-based prototyping of microneedle arrays (Figure detail B, right).
- Split-cavity molds allow complex 3D geometries. Figure detail C shows a bone screw, rings, and various shapes for oral drug delivery. Depending on the geometry of the prototype, single pieces in round or elongated molds and multi-cavity molds are possible. The VitalDose example shows six rings of various sizes made in one chamber.
- Multi-compartment free-d molding was tested to replicate an example demonstrated by Triastek® (Figure detail d). The multi-compartment tablet contains different segments to load different formulations. Multicavity molds allow for making multiple pieces from one formulation per molding cycle. Subsequently, the individual components are assembled manually and fused via VCM to obtain the multi-component prototype.
CONCLUSION
3D printing is transitioning from an emerging technology to a robust manufacturing technique for next-generation pharmaceuticals with complex geometries. The novel free-d molding technique can enable challenging 3D printing projects right in the beginning. New geometries can be explored with minimum material knowledge and resources. This significantly increases the chances of success. Once the formulators meet the desired quality attributes, the project can move toward process development.
REFERENCES
- Maniruzzaman, M. 3D and 4D Printing in Biomedical Applications, Weinheim, Germany: Wiley-VCH Verlag (2019)
- Shadambikar, G. VCM as a Screening Tool to Investigate Carrier Suitability for HME, Pharmaceutics, (2020)
- Repka, M. 3D Printing: Emerging Tech. and Functionality of Polymeric Excipients in Drug Product Dev, Springer (2024)