Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Food, Pharmaceutical & Bioengineering Division
- Metabolic Engineering I - General topics
- (264d) Membrane Surface Area Constrains Cell Maintenance Energy Generation

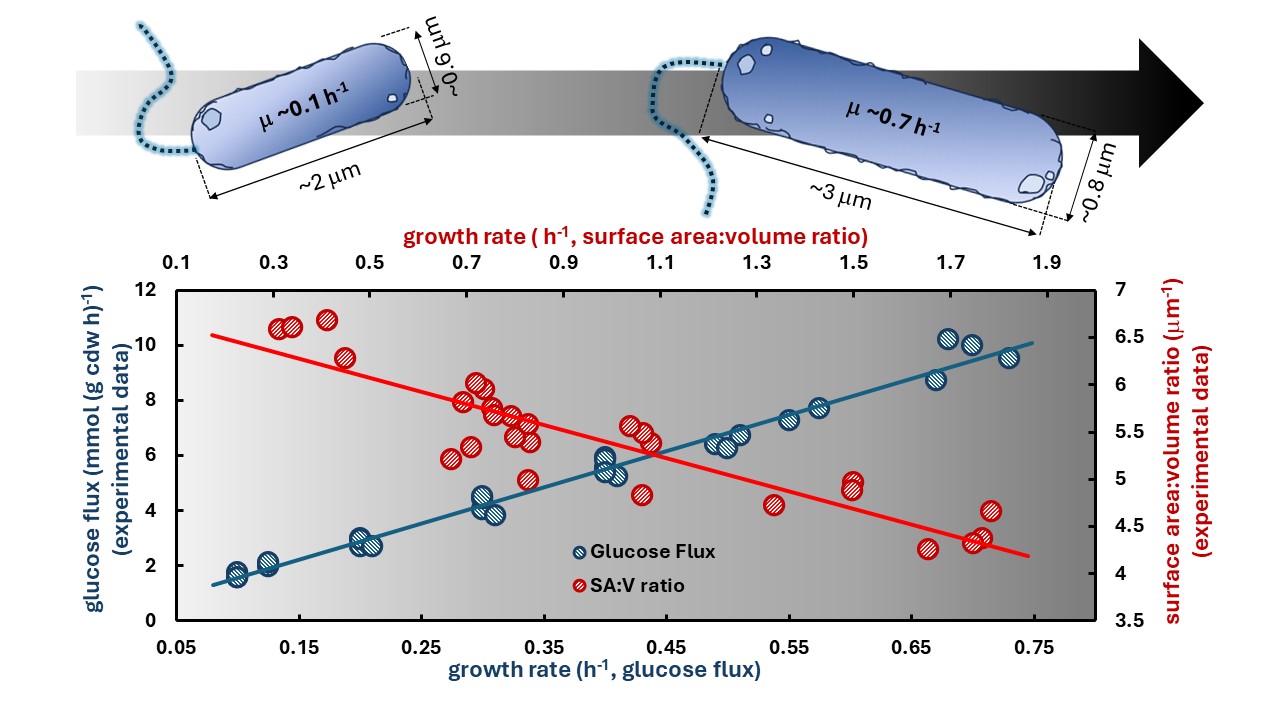
Bacterial cell geometry constrains both the surface area and the volume available for metabolic enzymes. Additionally, the surface area to volume ratio changes with growth rate, altering the availability of surface area for essential processes including nutrient acquisition, generation of a proton motive force, and ATP synthesis. We applied a new in silico systems biology theory to quantify constraints imposed by cell geometry on the potential for maintenance energy generation. Experimental data from Escherichia coli cultures grown under glucose-limited, ammonium-limited, or iron-limited conditions were analyzed for maintenance energy fluxes. Applying a membrane surface area constraint suggests the commonly assumed linear relationship between maintenance energy and growth rate is inaccurate. Glucose-limited cultures had a nonlinear, positive relationship between growth rate and maintenance energy, iron limited cultures had a negative relationship between growth rate and maintenance energy, while ammonium-limited cultures had a hybrid relationship combining aspects of the glucose- and iron-limited trends. Ergo, duly accounting for cellular geometry could alter commonly held assumptions about bacterial metabolism.