2024 AIChE Annual Meeting
(263h) The Hidden Chokepoints: Exploring Gas Diffusion in the Codh/ACS Enzyme Complex Using Molecular Simulations
Authors
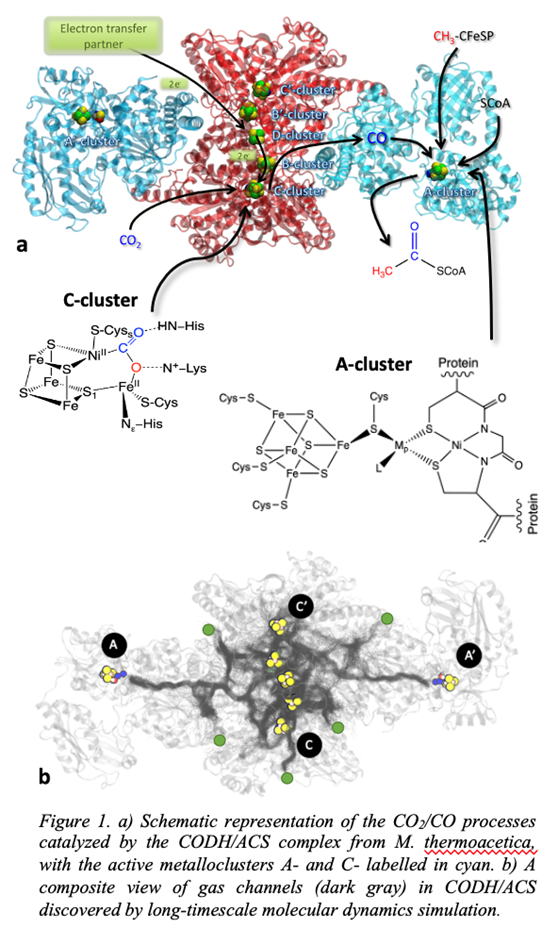
CODH and CODH/ACS are central for atmospheric carbon monoxide oxidation and supporting microbial life. These enzymes are also the focus of recent biotechnological applications because of their potential replacement of two energy-intensive industrial processes: 1) the water–gas shift reaction, a widely used industrial process for H2 or CO production, and 2) the Monsanto process used to produce acetic acid from methanol and CO. However, the effective and widespread use of CODH and CODH/ACS for industrial fields is limited by their poor activity under aerobic conditions. One strategy to design O2-tolerant complexes is to prevent O2 from entering the enzyme matrix.
Our research framework is centered on addressing key questions, 1) the reaction mechanisms at the organometallic active sites, 2) the transport channels of the gas molecules within the proteins, and role of protein conformations in the overall process, and 3) the rate of CO2/CO interconversion in each direction of the channels that enter or leave the catalytic cycle.
To address the fate of CO2 and CO upon reaching reaction sites, we employ density functional theory approaches to study the interaction of gas molecules at the active site within the protein environment. To elucidate the transport channels, we use atomistic free energy molecular dynamics simulations to explore the gas diffusion through the CODH/ACS complex from M. thermoacetica (Figure 1) and other design variants that favor uptake of CO2 and CO but restrict ingress of O2. We explore how the transport mechanism is affected by conformational changes in the subunits of the enzyme caused due to transformation of cavities and gating mechanism of residues. A hybrid Monte Carlo/Molecular Dynamics (MCMD) approach is used to investigate the electrocatalytic interconversions of CO2/CO at the active sites.