Water pollution is a major environmental concern worldwide. Dyes represent a major class of water pollutants. Numerous industrial sectors including textiles, food processing, paints and pigments, pharmaceuticals, and cosmetics produce substantial volumes of wastewater contaminated with dyes [1]. These dyes are characterized by their intricate aromatic structures and exhibit poor biodegradability [2,3], rendering them prone to bioaccumulation and subsequent contamination of aquatic food chains. As a result of these properties, dyes are designated as hazardous and carcinogenic water contaminants, posing significant risks to both human health and ecosystem integrity [4]. Accordingly, inadequate treatment of wastewater contaminated with dyes can lead to detrimental impacts on public health and environmental well-being [3].
Several techniques have been utilized for the treatment of dye-contaminated water. These methods include, but not limited to, sedimentation, flotation, coagulation-flocculation, filtration, adsorption, and advanced oxidation processes [5]. Among these techniques, adsorption has garnered substantial attention due to its advantageous attributes such as operational simplicity, versatility, high removal efficiency, cost-effectiveness, and scalability. However, adsorption efficacy greatly depends on the utilized adsorbent. Various types of adsorbents have been extensively investigated in the literature for their potential in efficiently removing organic dyes from wastewater effluents [3,6,7]. These adsorbents encompass modified activated carbons, graphene, carbon nanotubes, polymers, biochars, layered-double hydroxides (LDHs), zeolites, among others [5]. While some of these materials have exhibited promising adsorptive capabilities, some other have shown limited performance [8]. Hence, there is a pressing need for the development of more effective adsorbents in order to boost the efficacy of adsorptive dye removal from wastewater.
Accordingly, we report herein the synthesis of ceria nanotubes (CeNT)/zeolitic imidazolate framework-8 (ZIF-8) nanocomposite (abbreviated as CeNT@ZIF-8) and its application for the removal of a harmful azo dye (i.e., methyl orange) from contaminated water samples. In the synthesis process, 5 mL of 0.8 M Ce(NO3)3 aqueous solution was added to 75 mL of 6.4 M NaOH aqueous solution and stirred for 30 min. Then, the mixture was transferred to a 100 mL Teflon-lined autoclave and treated hydrothermally at 100 °C for 24 h. The obtained precipitates were washed using distilled water and ethanol alternatively for 3 times each and then dried. Subsequently, 0.26 g of the dried material was dispersed in 80 mL distilled water, followed by the addition of 3 mmol Ce(NO3)3. Then, the mixture was kept at 100 °C for 2 h. Afterwards, the obtained ceria nanotubes were extensively washed using distilled water and dried. Finally, the CeNT@ZIF-8 nanocomposite was prepared using the hydrothermal method where a certain amount of the produced CeNT was dispersed in a zinc aqueous solution before the addition of the organic linker (i.e., 2-methyl imidazole). The mixture was allowed to react for 1 h under stirring, followed by 1 h aging. The produced CeNT@ZIF-8 nanocomposite was, then, recovered and purified. For comparison, ZIF-8 was produced using the same method, but without the addition of CeNT. The synthesized nanocomposite and its parental materials (i.e., ZIF-8 and CeNT) were characterized using BET surface area, X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). These characterizations confirmed the successful synthesis of the CeNT@ZIF-8 nanocomposite.
After the successful synthesis of the CeNT@ZIF-8 nanocomposite, its performance in the adsorptive removal of MO was evaluated and benchmarked to the performance of the pristine ZIF-8 and CeNT. As displayed in Figure 1, the CeNT/ZIF-8 nanocomposite outperformed CeNT and ZIF-8 in the adsorptive removal of MO. For instance, the adsorption capacity of MO onto the nanocomposite is more than 18 and 7 times higher than those obtained using CeNT and ZIF-8, respectively. The superiority of the CeNT/ZIF-8 nanocomposite likely stems from the optimized interaction of MO with this adsorbent via potentially various mechanisms such as electrostatic, π−π stacking, hydrogen bonding, and hydrophobic interactions.
After demonstrating the superiority of the CeNT/ZIF-8 nanocomposite, other investigations were performed using the nanocomposite. These investigations include effect of contact time (i.e., adsorption kinetics), the effect of the initial MO concentration (i.e., adsorption isotherm), and the effects of pH and temperature, in addition to the regenerability and reusability of the CeNT/ZIF-8 nanocomposite. The experimental data obtained from the adsorption kinetics of MO onto the CeNT/ZIF-8 nanocomposite were fitted using the pseudo-first order (PFO) and the pseudo-second order (PSO). The fitting results revealed that the PSO tracks the experimental data better than the PFO with respective coefficient of determination (R2) of 0.9916 and 0.9654. Additionally, the experimental isotherm data were fitted using the Langmuir and Freundlich adsorption isotherm models with the Langmuir model providing more accurate fitting. The maximum adsorption capacity (qmax) under adsorbent saturation conditions predicted using the Langmuir adsorption model was 425.5 mg/g. This value is higher than those obtained using many other adsorbents reported in the literature [3,6,7].
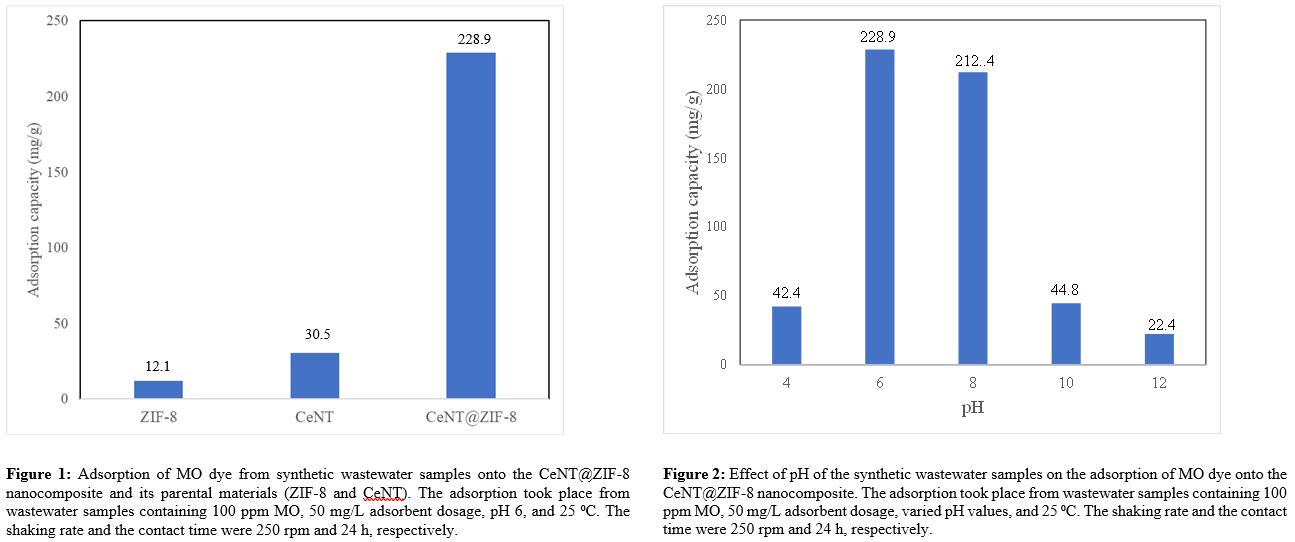
In addition to the adsorption kinetics and isotherm, the effect of pH on the adsorptive removal of MO using the CeNT/ZIF-8 nanocomposite was also investigated and the results are shown in Figure 2. The obtained results revealed that the optimum pH is 6 at which the MO adsorption capacity onto the CeNT/ZIF-8 nanocomposite from 100 ppm dye solution reached 228.9 mg/g. Below and above pH 6, MO adsorption declines, reaching 42.4 and 22.4 mg/g at pH 4 and 12, respectively (see Figure 2). Additionally, the thermodynamic investigation revealed that MO adsorption onto the CeNT/ZIF-8 nanocomposite is exothermic in nature. Furthermore, the reusability of the nanocomposite was also evaluated in this work.
Overall, the CeNT/ZIF-8 nanocomposite is a promising adsorbent for water decontamination. Nonetheless, further studies are still needed to explore the efficacy of this nanocomposite in the adsorptive removal of other organic and inorganic water pollutants. More importantly, the performance of the CeNT/ZIF-8 nanocomposite in treating real wastewater has to be thoroughly evaluated.
References
[1] G. Bal, A. Thakur, Distinct approaches of removal of dyes from wastewater: A review, Mater. Today Proc. 50 (2022) 1575–1579. https://doi.org/https://doi.org/10.1016/j.matpr.2021.09.119.
[2] S.A. Bahadi, M. Iddrisu, M.K. Al-Sakkaf, M.A.A. Elgzoly, Q.A. Drmosh, W.A. Al-Amrani, U. Ahmed, U. Zahid, S.A. Onaizi, Optimization of methyl orange adsorption on MgFeAl-LTH through the manipulation of solution chemistry and synthesis conditions, Emergent Mater. (2023). https://doi.org/10.1007/s42247-023-00513-z.
[3] S.A. Ganiyu, M.A. Suleiman, W.A. Al-Amrani, A.K. Usman, S.A. Onaizi, Adsorptive removal of organic pollutants from contaminated waters using zeolitic imidazolate framework Composites: A comprehensive and Up-to-date review, Sep. Purif. Technol. 318 (2023) 123765. https://doi.org/10.1016/j.seppur.2023.123765.
[4] M. Elzahar, M. Bassyouni, Removal of direct dyes from wastewater using chitosan and polyacrylamide blends, Sci. Rep. 13 (2023). https://doi.org/10.1038/s41598-023-42960-y.
[5] B. Sarwar, A.U. Khan, M. Aslam, A. Bokhari, M. Mubashir, A.A. Alothman, M. Ouladsmane, S.A. Aldossari, W.S. Chai, K.S. Khoo, Comparative study of ZIF-8-materials for removal of hazardous compounds using physio-chemical remediation techniques, Environ. Res. 220 (2023) 115168. https://doi.org/https://doi.org/10.1016/j.envres.2022.115168.
[6] S.A. Bahadi, Q.A. Drmosh, S.A. Onaizi, Adsorptive removal of organic pollutants from aqueous solutions using novel GO / bentonite / MgFeAl-LTH nanocomposite, Environ. Res. 248 (2024) 118218. https://doi.org/10.1016/j.envres.2024.118218.
[7] S.A. Bahadi, S.A. Bahadi, Q.A. Drmosh, Adsorption of Anionic and Cationic Azo Dyes from Wastewater Using Novel and Effective Multicomponent Adsorbent, Sep. Purif. Technol. 337 (2024) 126402.
[8] M. Zhenlin, T. DeZhi, Z. Hua, S. Asfandyar, A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water, Chem. Eng. J. 443 (2022) 136320. https://doi.org/https://doi.org/10.1016/j.cej.2022.136320.