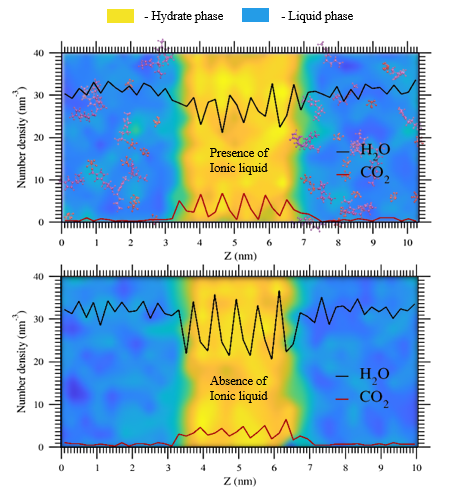
Ionic liquids (ILs) are considered environmentally benign, energy–efficient chemical inhibitors in the prevention of gas hydrate formation. Understanding the CO
2 hydrate dissociation in the presence of chemical inhibitors such as ILs is of importance; in preventing the CO
2 hydrate formation during CO
2 capture and sequestration as well as in the study of the combined injection method (i.e., CO
2 + chemical inhibitor injection) for methane extraction from gas hydrate reservoirs. As one of the steps towards this, in this work [1] we have performed molecular dynamics (MD) simulations to study the dissociation of CO
2 hydrates in the presence and absence of the ionic liquid, viz., [BMIM][PF
6]. Selection of this particular IL was made as Cadena
et al. in their MD simulations study demonstrated that CO
2 strongly associates with the [PF
6]
- anion and orients itself in a “tangent”-like configuration around the anion to maximize favourable interactions. [2] However, for the hydrate - liquid water - liquid carbon dioxide phases, experiments by Shin and coworkers demonstrated that a hydrophobic IL, viz., [BMIM][PF
6], does not contribute to carbon dioxide hydrate inhibition; which has been explained based on affinity between water and the IL. The hydration energy of [PF
6]
- has been determined to be approximately zero, leading to the ion not being solvated by water. Hence, the hydrogen bonds in the hydrate cages are not easily disrupted by [BMIM][PF
6]. [3] Therefore, to extend our understanding, in this work all-atom forcefield MD simulations of the CO
2 hydrate phase in contact with the aqueous phase (similar to the methodology used by Sarupriya and Debenedetti [4]) have been carried out in the NPT ensemble, at temperatures of 250, 255, and 260 K and over a wide range of pressures i.e., 20, 30.5, 37, 42, 50, and 60 bar. The choices for pressure in this study are the experimentally prescribed pressure values reported by Servio and Englezos [5]. Temperature values are near the melting point of ice described by TIP4P/2005 water forcefield. From the hydrogen bonding and order parameter analyses, the effect of [BMIM][PF
6] on CO
2 hydrate dissociation appears to be not significant. However, the local number density profiles of guest and host molecules bring out the microscopic effect of IL i.e., in the presence of this IL, deviation from hydrate-like behaviour is less than in its absence. This is due to the preferential association of CO
2 with [PF
6]
- anion. We observe an asymmetry in the local number density profiles of CO
2 and H
2O; the interface with filled partial cages exhibits a profile similar to hydrate-like whereas the interface with empty partial cages shows a deviated profile. This brings out the role of filled and empty partial cages at the interfaces on the CO
2 hydrate dissociation in the presence of [BMIM][PF
6]. The MD simulation-based approach used here can be further extended to identify ILs that hold the potential to affect the CH
4 hydrate dissociation and stabilize the CO
2 hydrates.
References:
[1] Chaudhury, A., Moorjani, B., Chatterjee, S., Adhikari, J. and Hait, S., 2023. Molecular insights into the dissociation of carbon dioxide hydrates in the presence of an ionic liquid, [BMIM][PF6]. Chemical Physics, 571, p.111943.
[2] Cadena, C., Anthony, J.L., Shah, J.K., Morrow, T.I., Brennecke, J.F. and Maginn, E.J., 2004. Why is CO2 so soluble in imidazolium-based ionic liquids? Journal of the American Chemical Society, 126(16), pp.5300-5308.
[3] Shin, B.S., Kim, E.S., Kwak, S.K., Lim, J.S., Kim, K.S. and Kang, J.W., 2014. Thermodynamic inhibition effects of ionic liquids on the formation of condensed carbon dioxide hydrate. Fluid Phase Equilibria, 382, pp.270-278.
[4] Sarupria, S. and Debenedetti, P.G., 2011. Molecular dynamics study of carbon dioxide hydrate dissociation. The Journal of Physical Chemistry A, 115(23), pp.6102-6111.
[5] Servio, P. and Englezos, P., 2001. Effect of temperature and pressure on the solubility of carbon dioxide in water in the presence of gas hydrate. Fluid phase equilibria, 190(1-2), pp.127-134.