2024 AIChE Annual Meeting
(187c) A Low-Iridium Anode for Durable PEM Water Electrolysis
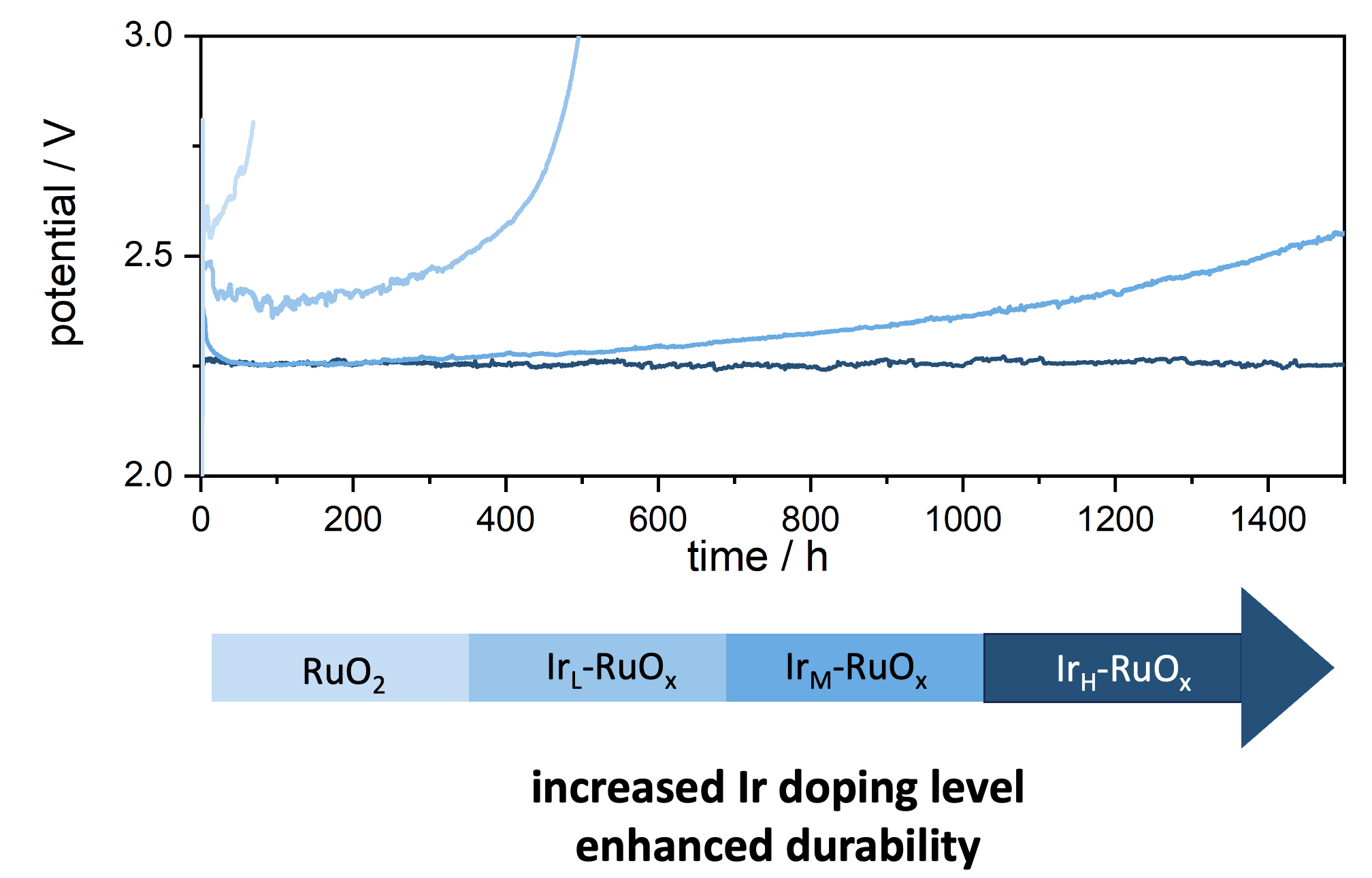
Herein, we synthesized a series of low-Ir doped RuOx (Ir-RuOx) with various Ir doping level, ranging from ~5 at% to ~15 at%. These catalysts have similar rutile structure and Ir atoms were found scattered homogeneously in RuOx lattice. The OER durability progressively improves with increased Ir doping, and IrH-RuOx successfully delivered outstanding stability of more than 1500 hours at industrial level current density without obvious degradation in a lab-scale PEM water electrolyzer. Metal dissolution rates exhibited a similar trend to the durability tests, validating the significantly suppressed Ru dissolution of Ir-RuOx. X-ray absorption spectroscopy confirmed the strong interactions between Ir and Ru, which is in good consistent with density function theory study results that the superior durability of IrH-RuOx originated from the stabilizing effects of Ir on adjacent Ru atoms. The IrH-RuOx was also tested by a third party collaborator in a larger scale PEM water electrolyzer device at 60 oC for more than 1000 hours and it performed lower degradation rate than commercial IrB catalyst, suggesting potential in future commercialization.