Water electrolysis is a promising strategy to produce H
2 gas for sustainable fuel and as a precursor feedstock to many chemical processes. Engineering electrolyzers that use anion exchange membranes and operate using impure water sources (i.e. seawater) offers a route to reduce water purification costs and unlock durable, affordable, and sustainable H
2 generation. Specifically, anion exchange membrane water electrolyzers (AEMWE) have been identified as an architecture that has the potential to compete with current carbon-intensive H
2 production methodologies. Seawater electrolysis in AEMs combines the key advantages of using cost-effective integral components (i.e. non-precious metal catalysts), generating high purity H
2, lowering ohmic losses, and utilizing alkaline solid electrolyte to suppress corrosive by-product formation (i.e., free chlorine). A major bottleneck for this technology is ionomer degradation that consequently results in device failure.
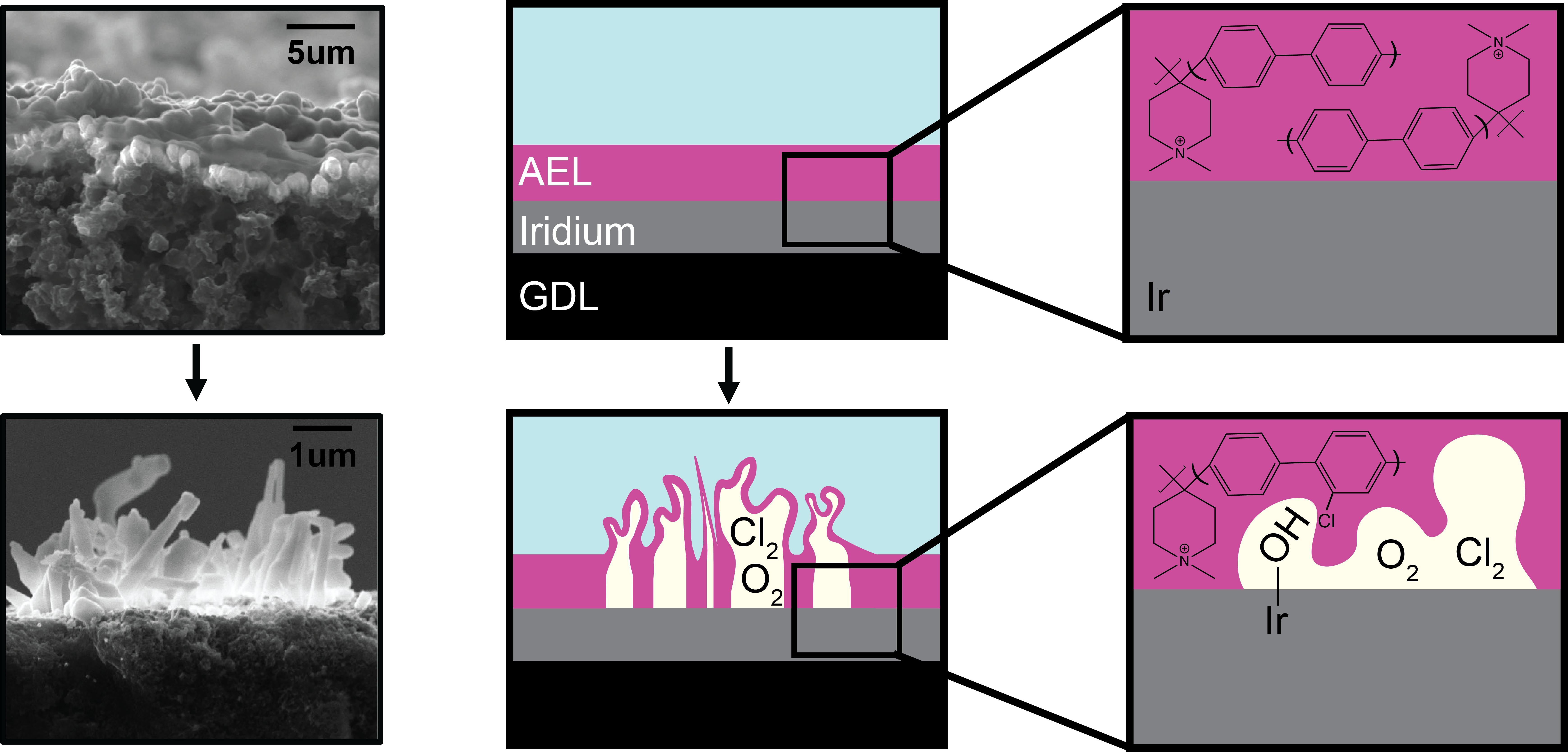
Here, we discuss the degradation of PiperION, a commonly used AEM, under anodic conditions and in the presence of ionic impurities (e.g, Na+, Cl-). These impurities, representative of those expected in impure water feeds (e.g. seawater) can compete with OER to generate corrosive free chlorine species (i.e., Cl2, HOCl, OCl-) at the anode. By investigating the electrochemical performance in the presence of these impurities and correlating them to the chemical decomposition products we gain insight to the decomposition mechanism of PiperION. When applying increasingly positive electrochemical potentials to the AEM, we observed changes in the polymer backbone, catalytic selectivity, and the electrochemically active surface area as a function of both current density and ionic concentration. Using X-ray photoelectron spectroscopy (XPS) and electrochemical mass spectroscopy (EC-MS), we detected the change in selectivity as well as products from ionomer decomposition. These insights allow for a better understanding of the ionomer/catalyst effects that are present in water electrolyzer that impact device lifespan and inform the operating conditions necessary to maintain long-term performance. By understanding the chemical degradation products and mechanism of AEMs, our study supports the development of new, more durable materials, which are crucial for accelerating the large-scale production of sustainable H2 from seawater.