2024 AIChE Annual Meeting
(181m) A Study on Thermomechanical Properties of Jeffamine-Based Multifunctional Polymer Nanocomposites
Authors
Polymer nanocomposites are materials made of a polymer or a polymer blend as a matrix and a component with at least one dimension smaller than 100 nm as a nano-additive. These materials have been widely used in different applications, such as automotive parts, coatings, construction materials, aerospace, flame retardants, packaging, and optoelectronic industry [1-2]. Various nano-additives can be added to the polymers to endow them with extraordinary properties without processability deterioration [3]. For example, carbon nanotubes (CNTs) are commonly used in polymer nanocomposites since they can enhance physical, mechanical, thermal, and electrical properties [4]. Moreover, quantum dots (QDs) have gained considerable interest due to their optical, electrical, magnetic, and catalyst properties [5].
Epoxy resins are thermosets that can be used as a polymeric matrix. The epoxide polymerization can occur by using different diamines as a crosslinker [6]. Jeffamines are amine-terminated poly(propylene oxides) with Tg below room temperature that can be used for the fabrication of these polymeric nanocomposites [7]. In addition to Jeffamines, reactive diamines can be engineered and synthesized to provide us with specific functionalization, improving nano-additives dispersion by conjugation. In this study, Jeffamine-based polymeric nanocomposites containing CNTs and QDs are characterized.
Materials and Methods:
First, bisphenol A diglycidyl ether (BADGE) was heated at 55 ◦C until it melted. Then, BADGE and difunctional Jeffamine (MW=4000Da) were mixed at a 1:1 epoxy: amine stoichiometric ratio for 1 h at room temperature using a stir bar. Next, carboxylic acid functionalized multiwalled CNTs with concentrations of 0.25wt% and 0.5wt%, dissolved in dimethylformamide, were added to the mixture followed by adding 0.001wt% carboxylic acid functionalized CdSe/ZnS core-shell type QDs dropwise. After that, the mixture was sonicated for 1 h. The precursors were cast into the molds and placed in the oven for 12 h at 120 ◦C. Finally, the thermomechanical properties of these thermosets were characterized by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) using TA Instruments. Tg of different samples were measured by DSC according to the following procedure: heating up to 250 ◦C (5 ◦C/min), isothermal for 60 min, cooling down up to -80 ◦C (40 ◦C/min), and heating up to 250 ◦C (5 ◦C/min). For TGA, samples were heated from room temperature to 500 ◦C at a rate of 20 ◦C/min under N2 atmosphere. Also, samples were cut using Leica EM FC 7 cryo-ultramicrotome at -80 ◦C to be observed under Titan transmission electron microscopy (TEM). Small angle X-ray scattering (SAXS) was performed by Xenocs Xeuss.
Results:
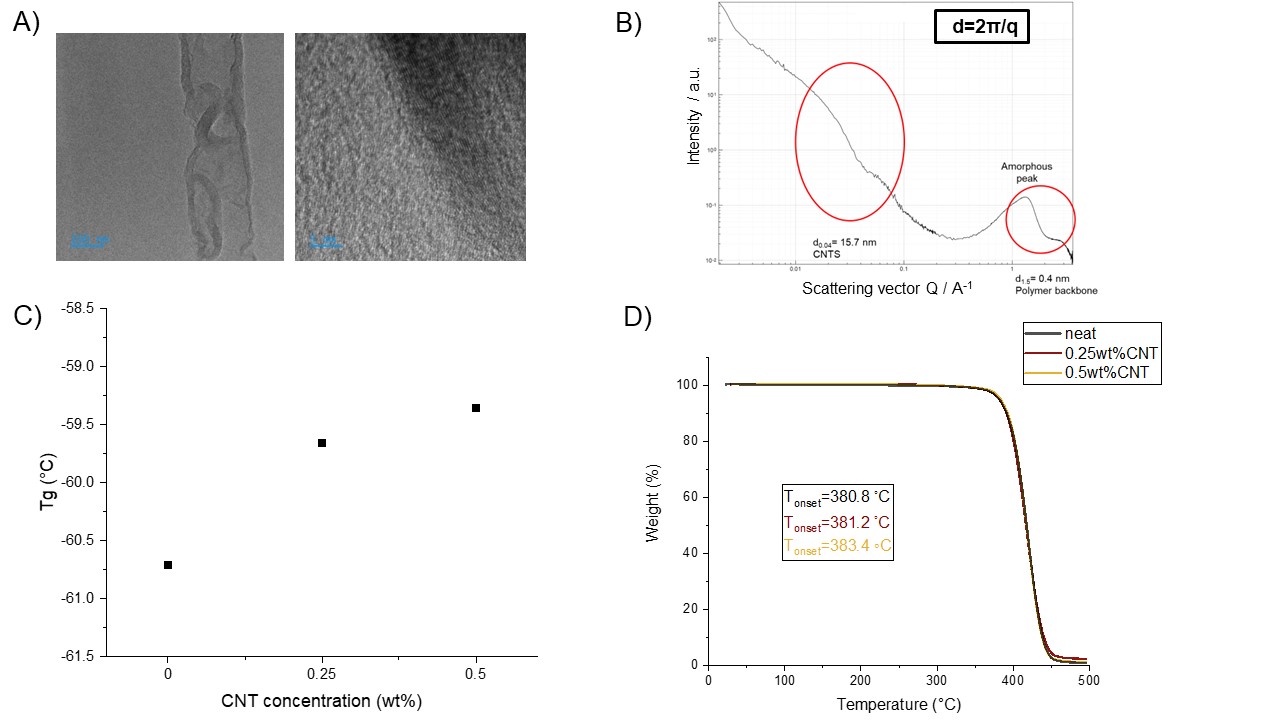
Jeffamine-based polymeric nanocomposites consisting of 0.001 wt% QDs and different concentrations of CNTs as well as neat samples without any nano-additives were fabricated. Figure 1A illustrates the dispersion of 0.25 wt% multiwalled CNTs within the epoxy resin. Also, an amorphous peak was observed and the presence of CNTs was confirmed by a hump at ~ 0.04 A-1 from the SAXS results in Figure 1B. The Tg of different samples evaluated by DSC as a function of CNT concentration is shown in Figure 1C. The samples containing CNTs had higher Tg compared with the neat sample which is attributed to the strong interface between CNTs and the polymeric matrix. The thermal stabilities of different samples were assessed by TGA (Figure 1D). The Tonset is the temperature at which the sample lost 5% of its mass. CNTs, in addition to having good thermal stability, restrict the mobility of polymer chains and therefore, prevent the degradation of polymeric nanocomposites.
Figure 1. Characterization of polymeric nanocomposites composed of bisphenol A diglycidyl ether and Jeffamine without nano-additives and with different amounts of CNTs (0.25wt% and 0.5wt%) and 0.001wt%QDs. (A) TEM images of samples containing BADGE+ Jeffamine+ 0.25 wt% CNTs showing CNTs dispersion within the matrix. (B) SAXS analysis of samples containing BADGE+ Jeffamine+ 0.25 wt% CNTs. (C) DSC results of thermosets with QDs and different CNT concentrations. (D) TGA results, illustrating the thermal stability of samples containing BADGE, Jeffamine, QDs, and different CNT concentrations.
Conclusions:
In conclusion, polymeric nanocomposites containing BADGE, Jeffamine, CNTs, and QDs were successfully fabricated, and their thermomechanical properties were characterized by TGA and DSC. Moreover, the presence and dispersion of CNTs within the polymeric matrix were verified and observed by SAXS and TEM analysis. In future studies, the thermomechanical properties of synthesized diamines will be investigated. Furthermore, the fluorescent activation and mechanoresponsive behavior of these composites for failure detection will be analyzed.
References:
[1] Winey K. I. MRS bulletin 2007;32(4):314-322
[2] de Oliveira, A. D. Nanocomposites-recent evolutions 2018:103-104
[3] Kumar, S. K. Macromolecules 2017;50(3):714-731
[4] Fu S. Nano Materials Science 2019;1(1):2-30
[5] Khan W. S. Science and applications of Tailored Nanostructures 2016;50
[6] Tjong, S. C. Materials Science and Engineering: R: Reports 2006;53(3-4):73-197
[7] Wang, M. Macromolecular Rapid Communications 2018;39(14):1800091