The increasing concentrations of atmospheric CO
2 emphasize an urgent need for effective strategies, highlighting the critical importance of sustainable solutions. Metal-CO
2 batteries offer a promising avenue for CO
2 capture and energy storage, yet challenges such as high overpotential, limited cyclability, safety, and cost hinder their progress. Despite the growing concern about carbon capture using safe and sustainable materials, nonaqueous Fe-CO
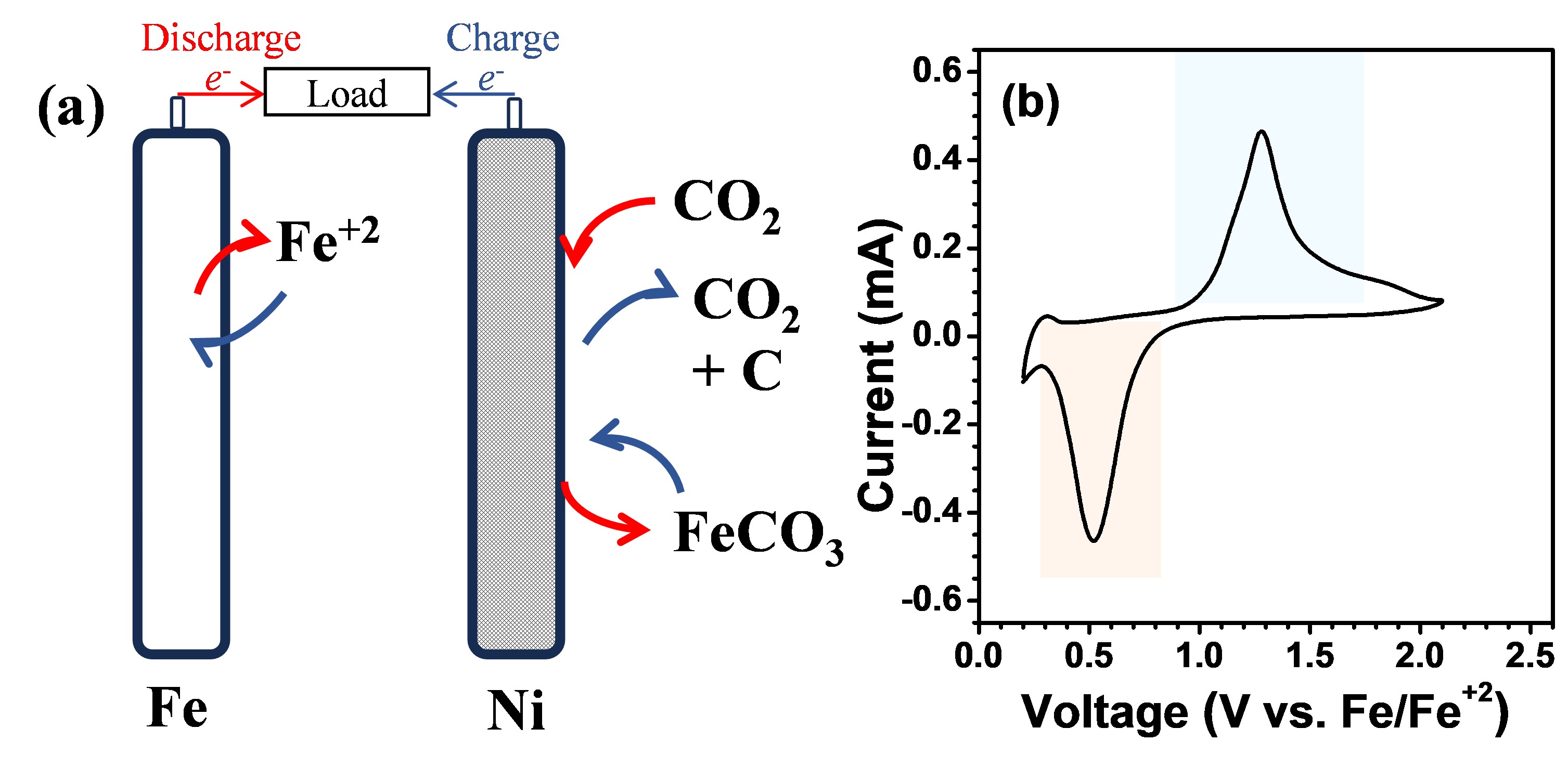
2 batteries have not yet been realized or investigated at the primary laboratory stage. Here, we develop a rechargeable Fe-CO
2 battery, enabled by a nonaqueous eutectic electrolyte i.e. 1M AlCl
3 in 1.7:1 [Et
3NH]Cl:FeCl
3. The cell demonstrates active redox chemistry in the potential window of 0.0-1.5V vs. Fe/Fe
+2 and delivers a high capacity of ~0.2 mAh/cm
2 at a current density of 80mA/cm
2. The cell sustains for >20 cycles, with a capacity retention efficiency of ~100%. The discharge product is investigated using the XRD and XPS analysis, confirming the formation of carbonates. This opens a sustainable avenue for rechargeable batteries to utilize and convert CO
2, while storing energy.