Biocatalysis is a sustainable method for production of a range of chemicals from high-value substances to commodity chemicals and biofuels. Enzymes conduct highly complex molecular conversions to produce specific products, making them an ideal tool that bypasses downsides of traditional chemical synthesis which requires energy intensive reactions, use of toxic chemicals or necessitates complex separations. As an alternative to metabolic engineering for biocatalysis, cell-free biocatalysis (CFB) has emerged to address limitations such as low yields due to side-reaction flux and restricted titers due to product toxicity. CFB has been demonstrated to work for even highly complex reaction cascades in an
in vitro environment. One such demonstration by Korman et al. (2017,
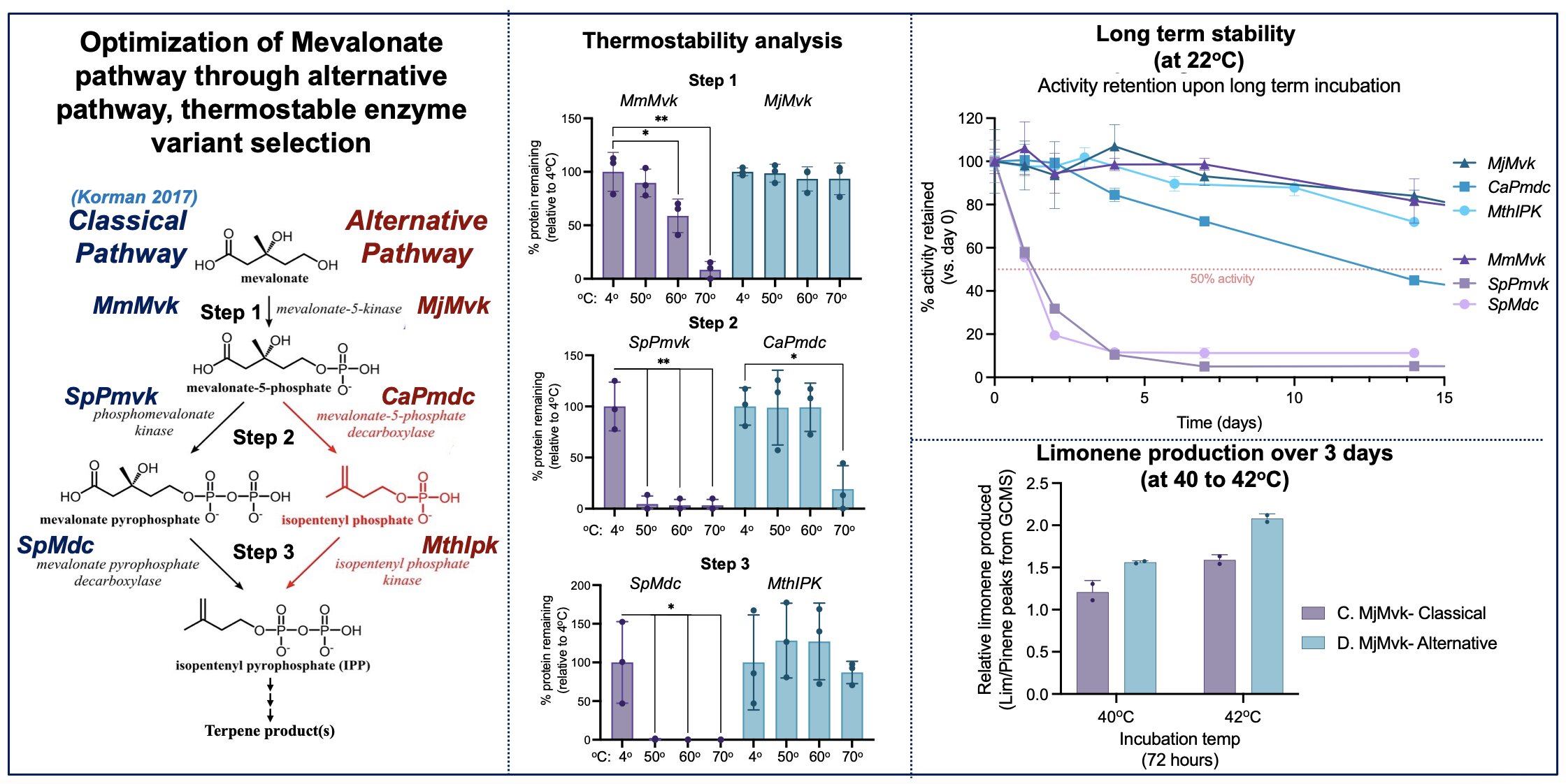
Nat. Comm.) showed high yields and titers producing various monoterpenes from starting glucose, by linking over 20 different enzyme conversions in a single pot. Here, we build off their work by addressing some universal CFB limitations to further ruggedize the lower mevalonate pathway enzymes. These three key steps, between mevalonate to isopentenyl pyrophosphate (IPP), serve as a gateway between glycolysis and hundreds of potential terpene products constructed from IPP building blocks.
CFB is fundamentally limited by enzyme operating lifetimes, as enzymes are inherently unstable and tend to lose activity over time. This work explores a hypothesized solution that thermostable enzymes, sourced from thermophilic organisms, will have longer operating lifetimes at moderate conditions. This will enable longer production runs to result in higher titers of CFB reactions. Towards this goal, we screened several enzyme variants sourced from thermophilic organisms along several characteristics such as expression level, thermostability, activity rates at 22oC, and long-term stability to select most optimal biocatalysts for this pathway. We found that thermostability correlated well with increase long term stability (at 22oC incubation) and activity retention for all variants tested. This marked a great improvement in theoretical operating lifetime over non-thermostable proteins which had been applied to cell-free synthesis of terpenes previously (Korman, 2017).
Running CFB reactions at supranatural temperatures is advantageous for enhancing enzyme reaction rates as well as mitigating any contamination of long-term reactions. Theoretically the alternative pathway of enzymes we’ve identified is better suited for long term reactions at elevated temperatures due to demonstrated thermostability as well as long-term stability. To probe this, we tested productivity of the classical pathway vs the thermostable variants we identified at 40oC and 42oC. Results confirm that indeed, the alternative pathway variants were more productive over a three-day limonene synthesis run. Altogether our data demonstrate that thermostable variants identified are more stable and productive to enable more robust mevalonate to terpene biocatalysis.