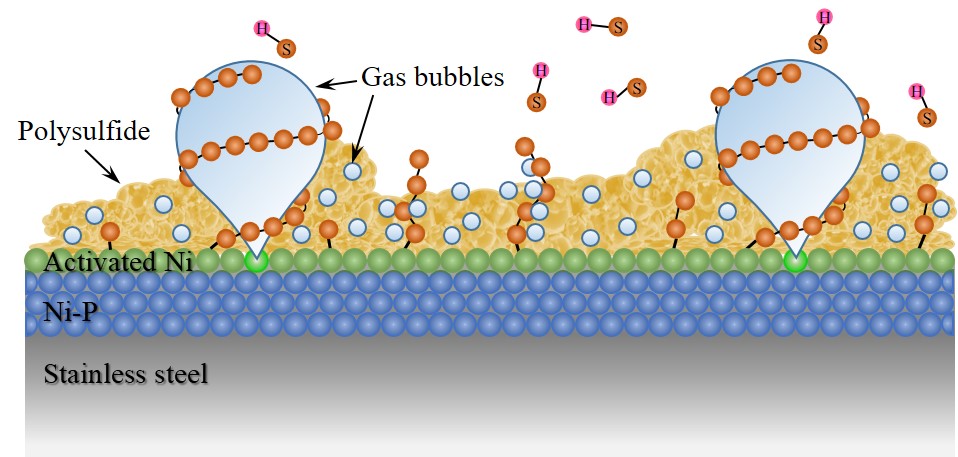
Hydrogen sulfide (H
2S) is toxic and widely exists in sulfide-containing waste and wastewater treatment processes, such as septic tanks, sewer systems for municipal wastewater, livestock manure storage pits, and anaerobic digestion, etc., causing odor issues and life-threatening problems. The concentration of H
2S in biogas varies from 50 ppm to 5000 ppm, while the Immediately Dangerous to Life or Health (IDLH) is 100 ppm. Removing H
2S to reduce work-related injury and environmental corrosiveness due to H
2S presence is critical work. Several methods, e.g., salt precipitation, adsorption, bio-scrubbing, and anode sacrifice, were developed for sulfide control in different scenarios. High sulfide removal efficiency has been achieved by these methods, but these methods tend to consume a large amount of chemical and energy. Alternatively, electrochemical sulfide oxidation is a promising approach for sulfide removal from wastewater or biogas due to its high sulfide removal efficiency, low energy, and chemical consumption. In this work, nickel phosphide was facilely electrodeposited on stainless steel (SS/Ni-P) and comprehensively evaluated as an anode catalyst for sulfide oxidation. Electrochemical property analysis suggested that SS/Ni-P had low polarization resistance (109.10 Ω) and high electrochemical active surface area (C
dl = 1.3 mF/cm
2). The sulfide removal efficiency of SS/Ni-P achieved 95.00 ± 3.55 % at 1.42 V
vs RHE within 48 h, which was 2.60 times and 3.62 times of that by SS/Ni and SS anodes, respectively. The first-order kinetic rate constant of SS/Ni-P for sulfide removal was 0.0315 ± 0.0061 h
-1, superior to the reported nickel-based catalyst with stainless steel substrate. Pre-treating SS/Ni-P under a constant external potential for 10 s further improved the electroactivity on sulfide oxidation due to the enhancement of the electrochemical active surface area by 46.56%. Further analysis of the chemical state changes during the sulfide oxidation process revealed that the Ni from Ni-P was the real active center, which formed Ni-S precursors to promote polysulfide formation. This work provided a highly efficient sulfide oxidation anode material for sulfide-containing wastewater and biogas treatment.