2024 AIChE Annual Meeting
(168c) Revolutionizing Nickel Extraction: Model Development for Optimal Hydrometallurgical Leaching of Laterite Ore
Authors
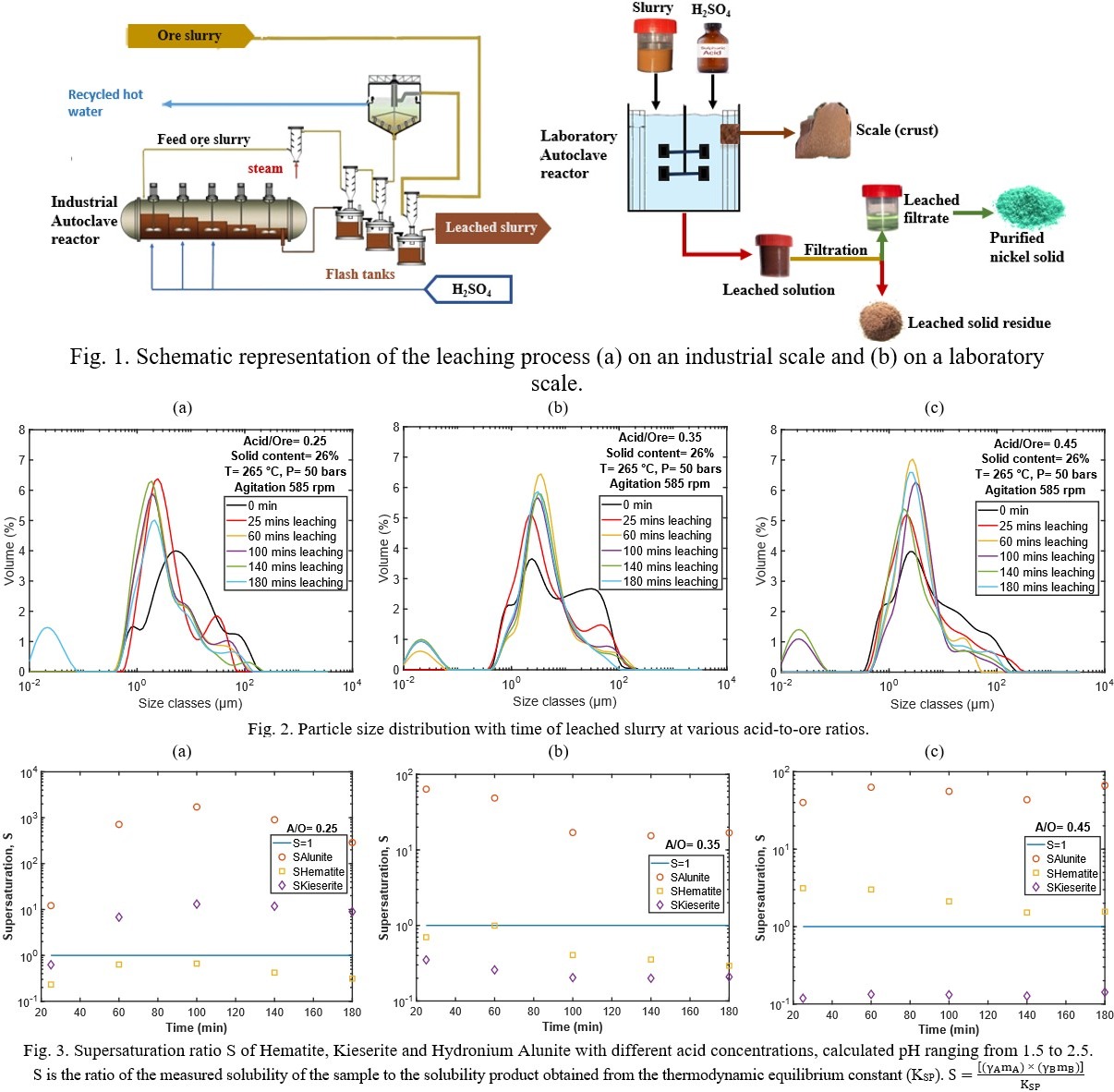
Atmospheric pressure acid leaching (AAL) and high-pressure acid leaching (HPAL) in continuous autoclaves have been extensively studied by researchers [1] for nickel extraction from lateritic ores. However, a common and significant challenge encountered in these hydrometallurgical processes is the formation of scales on the autoclave’s inner walls. These scales cause production downtime, limit the yield of Ni and consume energy as a result of descaling caused by frequent shutting down and restarting of the plant. This problem negatively impacts the environmental and economic aspects of the industry. Research shows that these scales, composed of minerals like hematite, kieserite, and alunite, form due to the high supersaturation ratios in the leached solution. The scales occur due to an increase of the supersaturation ratios in the autoclave, induced by the ore leaching and due to the sedimentation of formed minerals crystals in regions of low fluid velocity, and their cementation by crystal growth [2].
To study the leaching, and crystallization kinetics, and to optimize Ni extraction, an experimental set-up (HPAL) has been designed and commissioned. The equipment consists of a 0.5 L acid injection tank which allows the acid to be injected into the preheated slurry at a targeted temperature and a 2.8 L flat-bottom cylindrical autoclave tank made of titanium (grade 4) shell which is thermostatically controlled. The autoclave tank is equipped with a baffle to improve the circulation flow pattern of the suspension, and a magnetically driven Mixell-TT impeller (diameter = 0.7m) was used to keep the solids in suspension. For sampling, the leached samples are collected into a 20 mL steel-made sampling cell where the temperature and pressure of the sample are reduced to around 60°C and 1 bar by cold water circulating on the jacket of the cell before the sample collection.
The standard experiment was conducted at 265°C and a pressure of 50 bar in a sulphuric acid medium, with variations in the acid content at 585 rpm. Dry laterite ore powders from Prony Resources were used for the experiment, with an Acid/Ore mass ratio (A/O) of 0.25, 0.35 and 0.45 with an initial slurry solid content of 26 % in mass. Results show that the concentration yield of Ni in the liquid phase increases with leaching time with stability after 1 hr of leaching, and the extraction rate increases with higher acid concentration. However, this leaching rate influences the crystallization of solid particles: A new population of smaller particles (< 1 μm) appears as shown in Figure 2 due to mineral precipitation.
Understanding mineral solubility and chemical speciation in the liquid phase is essential for determining the concentration of complex ions that lead to crystal formation in the aqueous system. This speciation involves considering the equilibrium constants of reactions, and activity coefficients of chemical species. The first step of our work consists of the development of a speciation calculation model to predict the leaching process [3]. The model was first developed for temperatures between 200 – 300°C and considers the chemical equilibria of the important species in the aqueous solution which includes the following electrolyte species: H+, OH-, HSO4-, SO42-, Al3+, Al(SO4)30, AlSO4+, Fe3+, Fe2(SO4)30, FeSO4+, FeHSO42+, Fe(OH)2SO4-, Fe(SO4)2-, Mn2+, MnSO40, Mg2+, MgSO40, Ni2+, NiSO40, Co2+, and CoSO40. The developed speciation model utilized the equilibrium constant value of each chemical reaction extrapolated with the Density equilibrium constant model. The activity coefficients of the species were calculated from the B-dot (Truesdell-Jones) model [4]. To determine the supersaturation ratio of the scale-forming crystals, the model was applied to the experimental data for the hydrometallurgical Ni leaching system with ionic strength ≤ 2. Figure 3 shows the results obtained for the supersaturation ratios calculated for three solid species (Hematite, Kieserite and Hydronium Alunite).
In a second step, a second model was developed to take into account the dissolution of initial solids contained in the ore such as goethite FeOOH in the first step and the crystallization (nucleation and growth) of solids, such as Hematite Fe2O3, Kieserite MgSO4.H2O or Hydronium Alunite H3O(Al)3(SO4)2(OH)6 in a second step. This model should be able to plan and control the process, to ensure a consistent extraction of nickel, and to maintain the desired product specifications while controlling the leaching rate and minimising crust formation. This model aims to validate the hypotheses concerning the mechanisms involved and to identify and parameterize the kinetic expressions based on experiments carried out with the reactor developed for the studies. The kinetic parameters are identified by solving a system of ordinary differential equations comprising population balances for each solid species that crystallizes, written in the form of moments, material balances, and leaching balances written by size class with our realized experimental data.
References
[1]. McDonald, R. G., and Whittington, B. I., 2008. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy, 91(1), 35–55. https://doi.org/10.1016/j.hydromet.2007.11.009.
[2]. Whittington, B. I., and Muir, D., 2000. Pressure Acid Leaching of Nickel Laterites: A Review. Mineral Processing and Extractive Metallurgy Review, 21(6), 527–599. https://doi.org/10.1080/08827500008914177.
[3]. Dickson, O. V., Deleau, T., Coquelet, C., Espitalier, F., Lombart, J., Tardy, A., and Lachaize, F., 2023. Speciation and reaction equilibrium constant modelling of aqueous hydrometallurgical systems at elevated temperatures: A review. Chemical Thermodynamics and Thermal Analysis, 11, 100117. https://doi.org/10.1016/j.ctta.2023.100117.
[4]. Liu H., Papangelakis V.G., Chemical modelling of high-temperature aqueous processes, Hydrometallurgy 79 (1) (2005) Art. no. 1, https://doi.org/10.1016/j.hydromet.2003.10.014.