The pharmaceutical sector brings value to society through the discovery and deployment of life-saving medicines. In recent years, the market boom of next-generation therapies against life-threatening diseases and the urgent demands for vaccines during the pandemic have pressured the industry in rapidly scaling up manufacturing capacity to meet societal needs. Nevertheless, manufacturers catering for these markets reported shortages and delays due to unforeseen demand trends and uncertainty in manufacturing capabilities of platforms still under development [1, 2]. In this space, there is an increasing need for tools that help assess the impact of underlying manufacturing uncertainties on investment planning and mitigate against them.
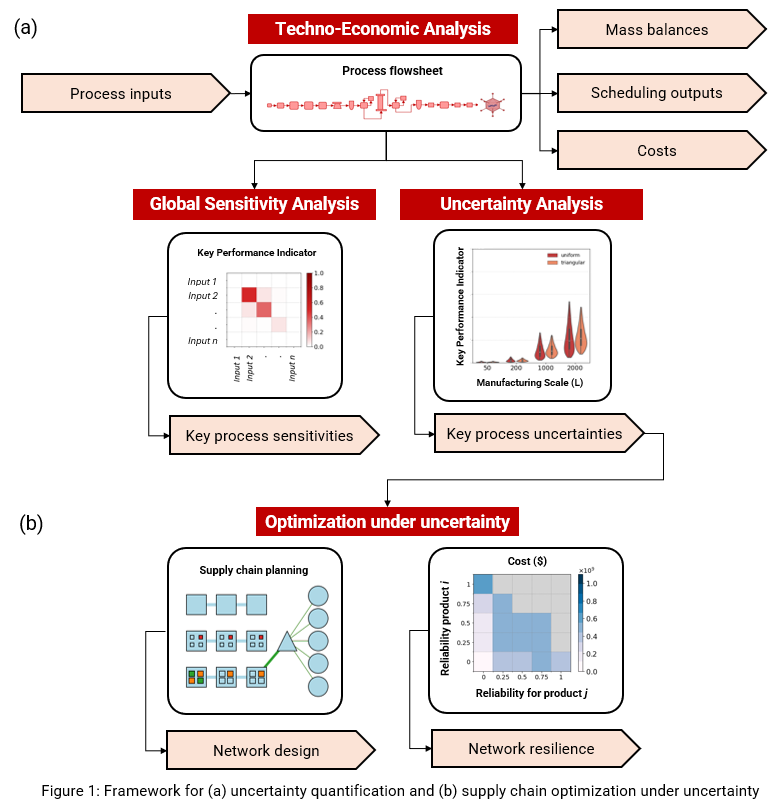
We present a decision-support tool for the quantification of supply chain resilience for networks that require rapid scale-up for commercialization and operate under supply-demand uncertainty (Figure 1). Firstly, process uncertainty is quantified via uncertainty analysis and global sensitivity analysis conducted on the developed techno-economic models for the studied product platforms [3]. Given the quantified probability distributions of process performance, a sampling-based approach is proposed to generate process performance scenarios. Secondly, a stochastic mixed-integer linear problem is formulated for the optimization of investment planning under process and demand uncertainty. Specifically, the scenario-based supply-demand constraint is formulated as a chance constraint. The probability of meeting the constraint effectively approximates a resilience metric for the overall supply chain. The accuracy of this approximation is tested via Monte Carlo-based simulations of manufacturing-related outcomes. Candidate investment plans for single- and multi-product systems are assessed, and cost-resilience Pareto frontiers are constructed for a range of chance constraint probability levels.
Firstly, results of the uncertainty and sensitivity analyses suggest a volumetric propagation of uncertainty with respect batch size and scale, which is primarily due to uncertainty in upstream process productivity. Critical sensitivities for all key performance indicators with respect to input parameters are also identified and output uncertainty distributions are recorded. Secondly, the optimization results highlight that for each target demand mitigating against all process uncertainties outcomes requires larger manufacturing capacity and cost. As system reliability is decreased, cost decreases and smaller scales are selected, as they meet demands with lower probabilities and at lower cost. This analysis also allows the quantification of maximum reliability of the system under limited capacity. Finally, we showcase how the presented framework can be augmented to incorporate sustainability metrics, to support the quantification of trade-offs amongst cost, environmental footprint, therapy availability and financial risk.
[1] Lambrix E, Kent-Egan H (2022) Viral vectors: shortages and innovation. In: Biotech Review of the Year
[2] Capra E, Gennari A, Loche A, Temps C (2022) Viral-vector therapies at scale: today’s challenges and future opportunities
[3] Sarkis M, Shah N, Papathanasiou MM (2023) Characterization of key manufacturing uncertainties in next generation therapeutics and vaccines across scales. J Adv Manuf Process. https://doi.org/10.1002/amp2.10158