2024 AIChE Annual Meeting
(158f) Engineering Membrane-Based Ion-Ion Separations for Sustainable Lithium Recovery
Authors
The U.S. Department of Energy classifies lithium as a critical mineral beyond 2025 due its high importance in the energy sector and high supply risk (Bauer, 2023). Given the economic and environmental implications of a strained lithium supply, lithium recovery from unconventional water sources such as produced water (an oil and gas waste stream), geothermal brines, and battery recycling is critical for the expansion of low-carbon and renewable energy technologies (Ventura, 2020; IEA, 2022; Standard Lithium, 2023). Both brines and battery leachate are complex waste streams, requiring a highly lithium-selective recovery process for direct lithium extraction. Membranes are an ideal candidate separation technology because of their energy efficiency and low chemical input relative to other techniques (solvent extraction, precipitation, adsorbents) (NASEM, 2019). For membrane-based processes such as selective electrodialysis (S-ED) to be suitable for lithium recovery, the development of highly lithium-selective membranes is required (Cath, 2021).
As it stands, commercial ion exchange membranes are size- and charge-selective but are not yet ion-selective. The permselectivity (ratio of competing ion transport rates across a membrane) between like-charged ions such as lithium and sodium in traditional membranes is negligible (~1). For lithium recovery from brines and battery waste via S-ED, a 1-2 order of magnitude improvement in ion permselectivity is required. A promising strategy to achieve ion-specific selectivity involves the incorporation of ion-coordinating ligands into membranes that have ion-specific interactions beyond general size sieving and electrostatic interaction. In this work, we seek to elucidate structure-performance relationships in novel, ligand-functionalized membranes that can inform ion-selective membrane design,
In addition to membrane chemistry, driving forces can also influence ion permeabilities and permselectivities. Most studies investigating new membrane chemistries for ion separations limit testing to one set of conditions – diffusive, concentration-driven transport. In addition to performing diffusion-driven studies for probing ion transport fundamentals, we also studied the effect of additional electric-potential driving forces on ion separations (permeability and permselectivity) relevant to S-ED. We also investigated the potential for selective up-concentration of lithium using a draw solution (e.g., sodium chloride) via Donnan Dialysis as an alternative driving force for selective lithium recovery.
Methods
To better understand ligand effects on ion transport and permselectivity, this work involves synthesis and testing of a library of ligand-functionalized membranes. Although previous simulation work studied the effect of ion-ligand interaction free energy on ion permeabilities, the effect of ligand density within the membrane has not been investigated. Our testing of membranes with variable charge density, ligand species and grafting densities provides a clearer picture of membrane structure-function relationships pertinent to ion-selective separation applications.
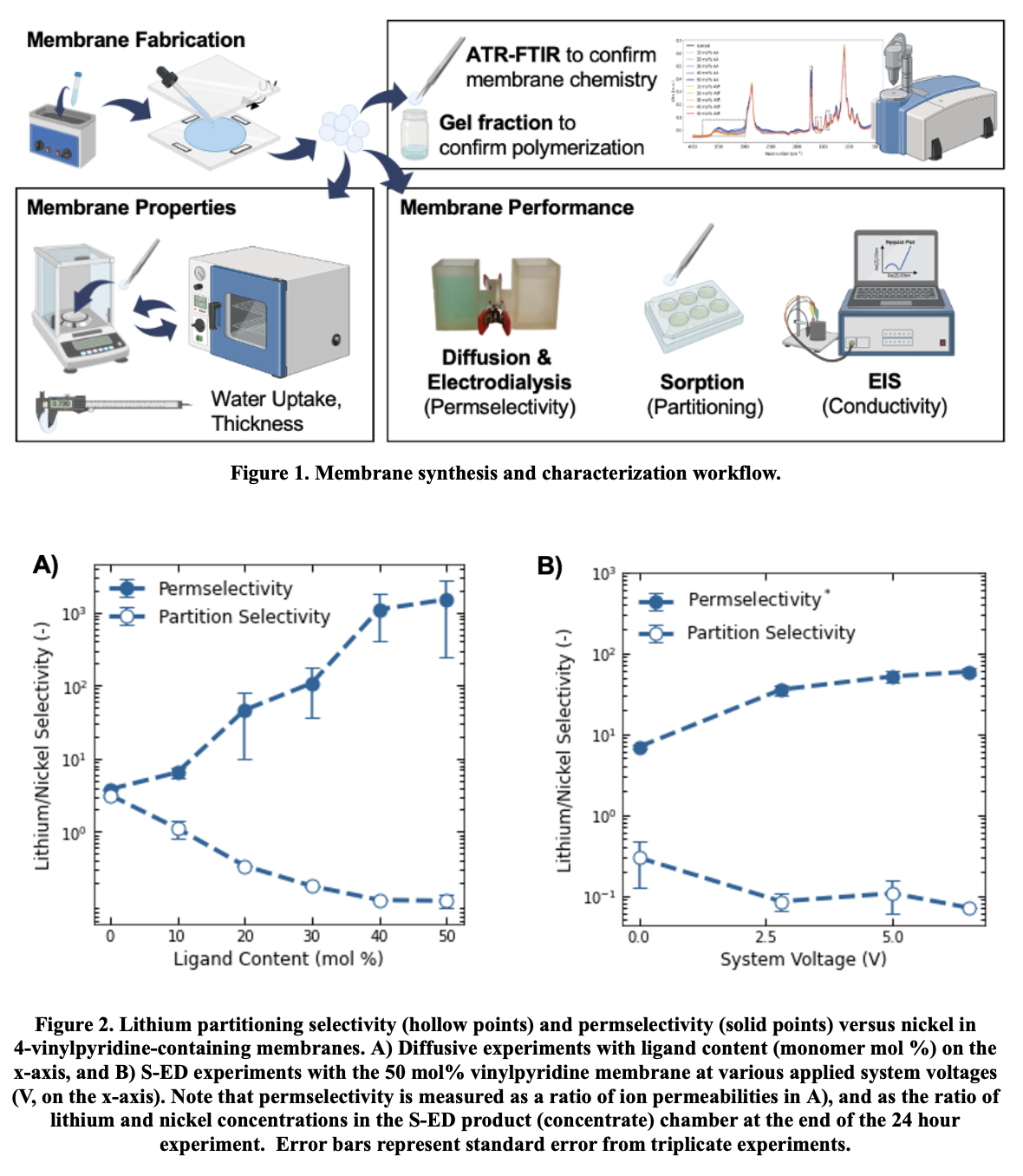
A comprehensive membrane synthesis and characterization workflow for the fabrication of polymeric membranes with controlled properties (e.g., chemistry, thickness, water content) has been established for this work (Figure 1). The workflow comprises free-radical polymerization for membrane synthesis, and gel fraction measurements, ATR-FTIR spectroscopy, and water uptake measurements for composition analysis. Membrane performance testing includes sorption measurements via ICP-OES, ion mobility measurements via electrochemical impedance spectroscopy, and ion permselectivity via diffusion and electrodialysis experiments in custom-made H-cells. These experiments were performed with simulated feedstocks relevant to lithium-rich brine and battery leachate (e.g., simplified, equimolar solutions of LiCl/NaCl, LiCl/MgCl2, LiCl/NiCl2).
Results & Discussion
Preliminary results from this work show that ligand-functionalized membranes can achieve significant ion-ion selectivity of critical material separations, with ligand density as a critical design variable (Figure 2A). One pyridine-functionalized membrane shows an order of magnitude improvement in diffusive lithium/nickel selectivity compared to commercially available cation exchange membranes, and subsequent S-ED results for operating at varying potential suggests that the additional electric potential driving force can enhance both lithium permeability and permselectivity compared to the diffusive case (Figure 2B).
In Donnan Dialysis applications, we have developed a thermodynamic model predicting lithium up-concentration and have performed bench-scale experiments validating predictions with controlled feed and draw solutions. Preliminary results show promising 24-28× lithium up-concentration with lithium/magnesium selectivity ratios up to 20 using a commercially-available cation-exchange membrane with sodium chloride or sodium bicarbonate draw solutions.
Conclusions and Implications
Ion-selective separations are critical in all stages of the lithium battery lifecycle: lithium extraction and refining, lithium battery use, and end-of-life recycling. The study of membrane-based ion transport in each of these fields will inform ion-selective membrane design under a range of environments and operating conditions. The resulting insights will support the sustainable, circular development of the lithium economy.
In this work we achieved meaningful enhancements in lithium permselectivity across novel membranes for S-ED applications, and have demonstrated selective up-concentration of lithium in a Donnan Dialysis application. Future work will investigate ion transport and energy input trade-offs across a range of operating conditions, shifting towards more realistic feed stream compositions to quantify process productivity, selectivity, energy consumption, and lifetime. Together, this work will identify quantitative technical targets for membrane-based ion-ion separations to be competitive in direct lithium extraction from brines and battery waste, supporting sustainable lithium recovery and global net zero emissions progress.
References
- The National Academies of Sciences, Engineering, and Medicine. A Research Agenda for Transforming Separation Science; 2019. https://web-s-ebscohost-com.stanford.idm.oclc.org/ehost/ebookviewer/ebo… (accessed 2023-11-09).
- Bauer, D.; Nguyen, R.; Smith, B. Critical Materials Assessment; U.S. Department of Energy, 2023. https://www.energy.gov/sites/default/files/2023-07/doe-critical-materia… (accessed 2024-02-01).
- The Role of Critical Minerals in Clean Energy Transitions; World Energy Outlook Special Report; International Energy Agency, 2022; p 287.
- Standard Lithium Ltd. Arkansas Smackover. Arkansas Smackover: Standard Lithium Ltd. https://www.standardlithium.com/projects/arkansas-smackover (accessed 2023-11-09).
- Ventura, S.; Bhamidi, S.; Hornbostel; Nagar, A. Selective Recovery of Lithium from Geothermal Brines; 2020.
- Cath, T. Y.; Chellam, S.; Katz, L. E.; Breckenridge, R.; Cooper, C.; Ellison, K.; Macknick, J.; McKay, C.; Miller, K.; Monnell, J.; Rao, N.; Rosenblum, J.; Sedlak, D.; Stokes-Draut, J. National Alliance for Water Innovation (NAWI) Resource Extraction Sector Technology Roadmap 2021; DOE/ GO-102021-5567; National Renewable Energy Lab. (NREL), Golden, CO (United States), 2021; pp 87–93. https://doi.org/10.2172/1782446.