In recent years, the field of materials science and engineering has witnessed a growing interest in nanoscale materials with unique physical, chemical, and mechanical properties. These materials, derived from easily accessible, cost-effective, and renewable precursors, hold enormous potential for applications in surface coatings, biomedicine, environmental catalysis, pharmaceuticals, and renewable energy [Wu M., 2020].
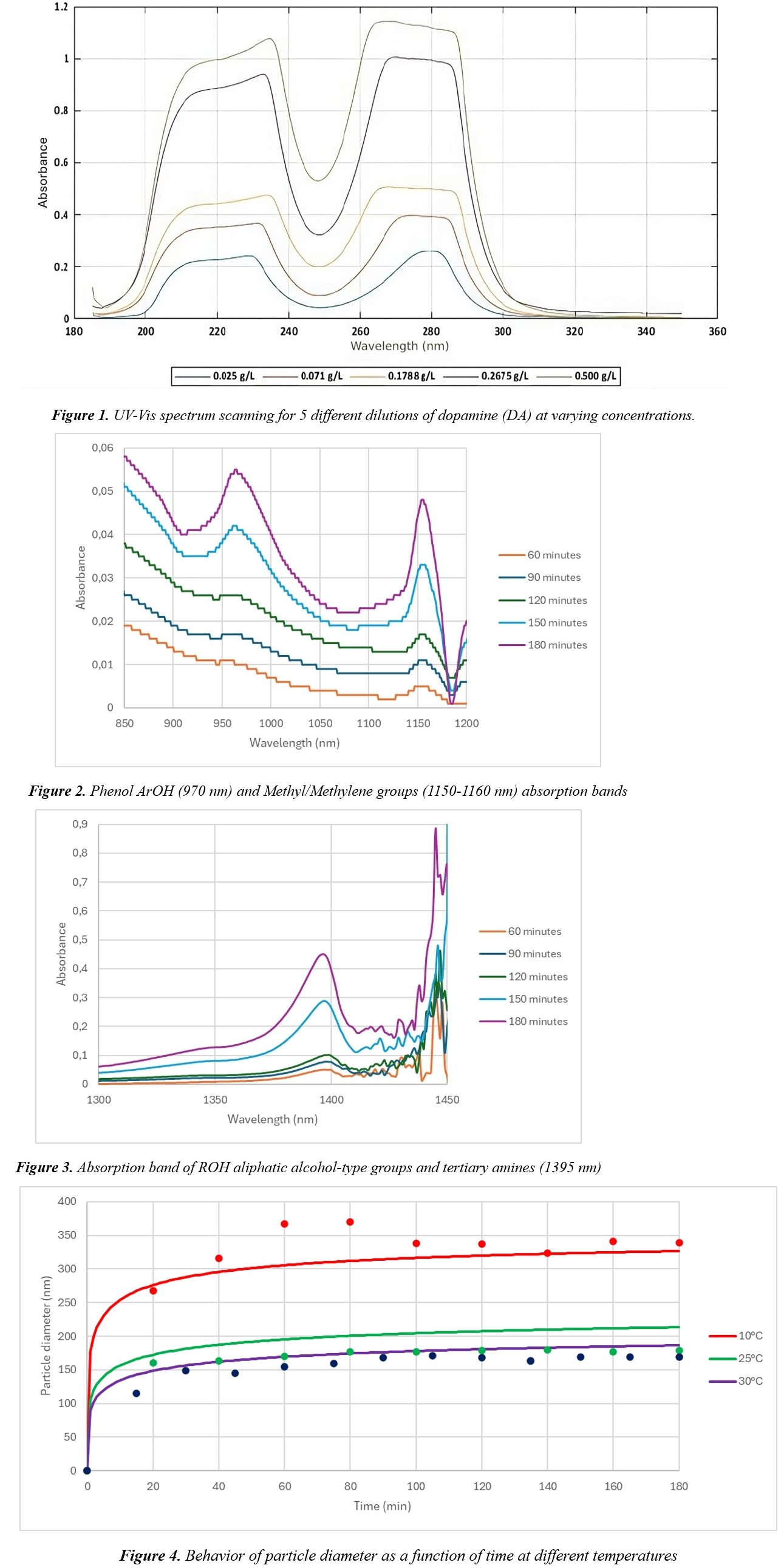
This study investigates the kinetic behavior of dopamine (DA) polymerization through auto-oxidation in the presence of air to produce polydopamine (PDA) nanoparticles. The synthesis of PDA nanoparticles is crucial due to their potential applications in various fields. Specifically, PDA nanoparticles have been explored to remediate contaminated water, develop pharmaceutical products, and effectively treat diseases such as cancer. Additionally, they offer solutions for sustainable energy storage. Since nanoparticle properties exhibit distinct characteristics based on their size, this study focuses on examining the influence of reaction temperature and alkali concentration on the average diameter of PDA nanoparticles. [Djermane, R., 2021] Based on experimental data, a model is proposed to describe the variation in nanoparticle diameter as a function of temperature and alkali concentration, considering that the Avrami model proposed as an initial model does not show a direct relationship between the kinetic constant and temperature.
Furthermore, it is suggested to utilize near-infrared spectroscopy (NIR) and UV-Vis technique as tools for monitoring and analyzing this reaction. These techniques aid in comprehending the physicochemical properties of the reaction by detecting the formation and consumption of specific chemical groups throughout the process, identifying absorption peaks or characteristic bands in the spectrum [Pegueros Gutierrez A., 2010]. Specifically, UV-Vis allows determining the concentration of the compounds involved, with results presented in Figure 1, while NIR, with results such as those shown in Figure 2 and Figure 3, is employed to characterize and identify the properties of the compounds by recognizing specific peaks in the spectrum.
On the other hand, considering that current polydopamine formation reactions are conducted in batch processes, it is acknowledged that this mechanism entails operational disadvantages such as lower production capacity, in addition to potential challenges in maintaining consistent product quality and increasing production costs. Therefore, understanding the reaction kinetics paves the way for scalability from a batch to a continuous process, which is a crucial aspect with significant industrial implications. Given the importance of the size of polydopamine nanoparticles in obtaining properties with antineoplastic behavior, and their potential use as therapy in cancer, comprehending the influence of operating conditions on the properties of the nanoparticles is crucial for the implementation of continuous production processes [Nieto C., 2021].