Acetonitrile, being a xenobiotic that readily volatilizes into the atmosphere, poses a severe concern to the health of human beings and animals when it is released into the environment because it breaks apart into toxic hydrogen cyanide and acetaldehyde.
1 Consequently, acetonitrile is regarded as one of the essential contaminants in industrial wastewater, and its removal from water is important. Considering the toxicity of acetonitrile, molecular simulations (which are computer experiments) are a cost-effective alternative to physical experiments; and the results obtained will reduce the number of experiments to be performed to find efficient task-specific solvents for wastewater treatment. In literature, mixtures of acetonitrile and water, as also mixtures of alcohols with acetonitrile and/or water,
2,3,4,5,6,7 have been studied in single-box simulations performed in the isothermal isobaric ensemble. The novelty of this study (as compared to literature) is that it is on liquid-liquid equilibria (LLE) where, besides generating the coexistence data and computing the Gibbs free energies of transfer for acetonitrile from the aqueous to the organic phase; molecular insights obtained which characterize the features of the radial distribution function will permit us to identify efficient solvents as well as design new, green solvents. Towards gaining molecular insights into the use of methanol or ethanol as an extraction solvent to remove acetonitrile from its mixture with water, we have performed three-box GEMC simulations to model LLE using the molecular simulation technique pioneered by Keasler and co-workers.
8 The three thermodynamically connected boxes
8 represent the water-rich aqueous phase, the organic-rich phase, and a dummy vapour box containing helium atoms. The third box increases the rate of particle transfers between the two liquid boxes.
8 Total number of molecules for the entire 3-box system combined contains 1000 water, 300 acetonitrile, 300 ethanol/methanol, and 50 helium molecules. Water is modeled using the TIP4P/2005 force field, whereas acetonitrile, ethanol/methanol, and helium are modelled using the TraPPE-UA force field. For ethanol as the extraction solvent at 293.15 K and 101.325 kPa, the chemical potentials of all three species in the coexisting aqueous and organic phases are in good agreement establishing equilibrium has been attained with partition coefficient of acetonitrile in the organic and aqueous phases being 5.09. The Gibbs free energy of transfer for acetonitrile is -2172.45 J/mol; the negative value confirming that the transfer to the organic phase is favourable. We also note here that the specific density of the aqueous phase is 0.937 g/cm
3, and that of the organic phase is 0.807 g/cm
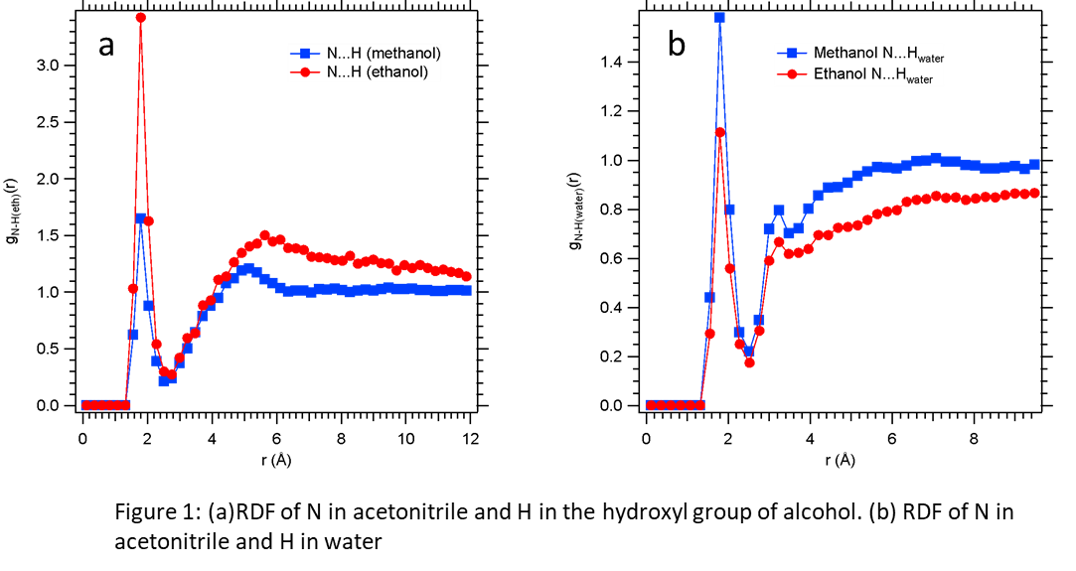
3. Our investigations show that ethanol is a better solvent than methanol (Gibbs free energy of transfer for acetonitrile is -141.47 J/mol) at 298.15 K and 101.325 kPa as methanol is more soluble in water and to understand the underlying physics, we compare the site-site intermolecular radial distribution functions in the organic-rich phase of both the systems. For the site-site RDF of N from acetonitrile and H of the hydroxyl group of alcohol, we find in Figure 1(a) that first peak occurs at 1.8 Å with ethanol peak height of 3.4 being much higher than the methanol peak height of 1.65, indicating more affinity of acetonitrile towards ethanol whereas the reverse is seen in the site-site RDF (Figure 1(b)) of N in acetronitrile with respect to the H of water. These are the characteristics that allow us to identify a better solvent for extraction of acetonitrile from water and will help in the subsequent molecular simulation based design of green solvents.
References
[1] Greenberg, Mark. "Toxicological review of acetonitrile." Katherine A, and David L. Risk Assessment Division. US Environmental Protection Agency Publishing, Washington, USA (1999): 25-31.
[2] Hloucha, M., A. K. Sum, and S. I. Sandler. "Computer simulation of acetonitrile and methanol with ab initio-based pair potentials." The Journal of Chemical Physics 113.13 (2000): 5401-5406.
[3] Satoh, Yasushi, and Koichiro Nakanishi. "Theoretical studies of acetonitrile water mixtures/monte carlo simulation." Fluid phase equilibria 104 (1995): 41-55.
[4] Sun, Li, J. Ilja Siepmann, and Mark R. Schure. "Monte Carlo Simulations of an Isolated n-Octadecane Chain Solvated in Water− Acetonitrile Mixtures." Journal of Chemical Theory and Computation 3.2 (2007): 350-357.
[5] Nagy, Peter I., Mugunthu R. Dhananjeyan, and Paul W. Erhardt. "Monte Carlo structure simulations for tertiary-butyl alcohol solutions in water/acetonitrile solvents." Journal of Molecular Structure: THEOCHEM 895.1-3 (2009): 116-126.
[6] Nagy, Peter I., and Paul W. Erhardt. "Monte Carlo Simulations of the Solution Structure of Simple Alcohols in Water− Acetonitrile Mixtures." The Journal of Physical Chemistry B 109.12 (2005): 5855-5872.
[7] Mountain, Raymond D. "Microstructure and hydrogen bonding in water− acetonitrile mixtures." The Journal of Physical Chemistry B 114.49 (2010): 16460-16464.
[8] Keasler, Samuel J., et al. "Molecular insights for the optimization of solvent‐based selective extraction of ethanol from fermentation broths." AIChE Journal 59.8 (2013): 3065-3070.