Catalytic dehydration of methyl lactate (ML) to synthesize methyl acrylate enables the use of biomass rather than petroleum-derived feedstocks. Here, we use kinetic studies,
in situ infrared
spectroscopy, and catalyst characterizations to probe the ML dehydration mechanism on ion-exchanged faujasite (FAU) catalysts (Na, K, Cs).
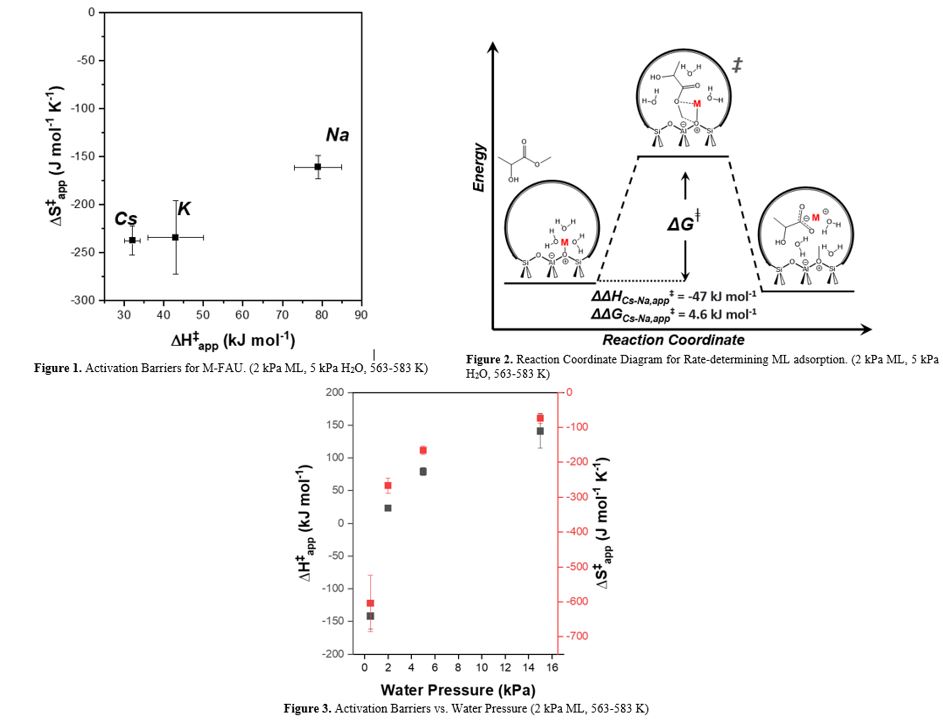
All M-FAU possess methyl acrylate rates that increase proportionately to ML pressure and appear independent of H2O pressure, which suggests a common mechanism and kinetically relevant step (0.5-10 kPa ML, 0.5-15 kPa H2O, 573 K). Activation enthalpies for methyl acrylate formation increase with cation size, which occurs concomitantly with activation entropy decreases (Figure 1). These trends correlate with volumetric water uptake, in which smaller cations associate with greater amounts of water. Figure 2 portrays a reaction coordinate consistent with these observations and supported by in situ infrared spectroscopy, which involves concurrent formation of a surface methoxy carbocation and an alkali metal lactate complex solvated by intrapore water upon ML adsorption. The enthalpy-entropy compensation across M-FAU gives rates that differ by a factor of 2 at standard conditions (2 kPa ML, 5 kPa H2O, 573 K). Ongoing work involves using calorimetry to measure heats of adsorption and relates these to enthalpic barriers. Figure 3 shows increases in the H2O pressure increase apparent activation enthalpies and the dependence of rates on ML pressure over Na-FAU. These differences reflect a shift in the dominant surface species and kinetically relevant step. Apparent barriers differ by more than 100 kJ mol-1 and rates evolve from inverse first-order to first-order dependence on ML pressure. These findings do not appear within the current literature and explain how the identity of alkali metal cations impact ML conversion rates and co-fed H2O pressure can change the operating kinetic regime by altering the pore microenvironment.